LIVE-CELL IMAGING/FEMTOSECOND LASERS: Ultrafast lasers + THG microscopy = flexible in-vivo cell research
Third-harmonic generation (THG) microscopy is attractive for long-term imaging on living organisms because it provides high resolution and requires no staining. Use of a femtosecond pulsed fiber laser at 1550 nm yields a signal in the visible range that is compatible with standard optics, and efficiently detectable with conventional photomultiplier tubes (PMTs).
ByRodrigo Aviles-Espinosa, Pablo Loza-Alvarez, Andreas Brodschelm, and Wilhelm Kaenders
Enormous efforts are being made to understand disease pathologies and to find ways to treat and prevent them. Studies on isolated cells and tissues are not able to mimic the metabolism of a complete organism, which involves a complex interplay between various tissues and multiple signaling pathways. For this reason, model organisms, such as the nematode Caenorhabditis elegans (C. elegans), are useful. The C. elegans worm is ideally suited for biomedical research because of its short life cycle, small size and transparency–factors that enable it to provide insights in several biological and physiological functions. Live microscopy techniques give insights into the processes involved in cell and tissue formation and enable systemic studies.
The standard approach to imaging the nematode's structure and monitoring its biological functions is fluorescence microscopy–which requires staining the areas of interest with dye. However, photobleaching and phototoxicity are fluorescence-related issues that often hinder long-term live-cell studies. Furthermore, the dyes used to label the specimens may potentially alter the observed structures and their function, and even be incompatible with live-cell imaging.
THG microscopy at 1550 nm
Recently, a collaboration between the Institute of Photonic Sciences (ICFO) in Spain and the German firm Toptica Photonics AG demonstrated the potential of third-harmonic generation (THG) microscopy for long-term time-lapse studies in highly sensitive developmental processes of C. elegans. The Spanish and German researchers used the FFS, a femtosecond pulsed fiber laser system by Toptica, to demonstrate the strong potential of THG at 1550 nm for high resolution 3D in-vivo time-lapse imaging.1
THG is a coherent, nonlinear imaging technique especially useful for live-cell studies: It provides intrinsic high resolution in three dimensions, enables optical sectioning and requires no labeling. Another coherent technique, for example, is second-harmonic generation (SHG), which requires non-centrosymmetric structures; therefore, SHG is limited to imaging collagen, myosin and microtubules.
Conventional THG systems are generally equipped with Ti:Sapphire lasers emitting wavelengths between 650 and 1100 nm, which yield a THG signal in the UV range (220-360 nm) and require dedicated UV-grade optics. Exciting a specimen at 1550 nm overcomes several drawbacks of standard THG systems. The generated signal is, at 516 nm, visible–and thus, the integral optics of a standard microscope system are optimal. Another advantage is that the 516 nm signal falls near the peak sensitivity region of conventional PMTs, allowing for very efficient signal collection.
You may wonder whether irradiation at 1550 nm leads to greater sample heating compared to shorter wavelengths, and impedes long-term live-cell imaging. The research shows that this is not the case: water absorption at wavelengths in the IR range does not facilitate sample heating if a sufficiently strong THG signal can be generated at very low average powers. Indeed, THG at 1550 nm allows long-term imaging even during the very sensitive stages of embryo development, yielding information from different tissues/structures and leaving the viability of the specimen uncompromised. In agreement with previous studies, this work has proven that THG imaging at 1550 nm can be advantageously used to follow processes in living unstained specimens for several hours.2-6
In-vivo studies of embryogenesis/morphogenesis
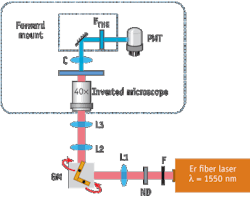
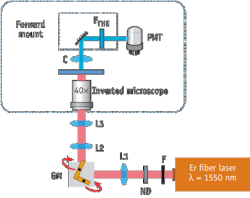
The research team used a standard inverted microscope equipped with a pair of x/y-galvanometric mirrors to work as a laser scanning microscope (see Fig. 1). For THG excitation, the compact fiber based pulsed laser system (FFS, Toptica Photonics) at 1550 nm ran with a pulse duration of 100 fs, a repetition rate of 107 MHz and an average output power of 350 mW. After passing through all the optical elements, the measured average power at the sample plane was 4.9 mW, which corresponds to 460 W peak power. A photomultiplier tube (PMT) mounted on a custom-made, forward-detection path detected the THG signal. In front of the detector, a bandpass filter around the central THG wavelength of 516 nm was used to separate the THG signal from the fundamental wavelength.
The researchers performed experiments on wild type (N2) C. elegans embryos and generated images of the different C. elegans stages–from cell division inside the hermaphrodite, namely gastrula, coma and multiple fold stages, to the L1 larva after hatching (see Fig. 2). The detected THG signal originates from different structures, depending on the stage of the nematode. In early stages, the signal is generated from individual cells, whereas in later stages, the signal arises mainly from the outer layers of the embryo. The signal generated from an individual cell shows the nucleus as a dark region due to the nucleus' homogeneous nature. During cell division stages, cells contain lipid depositions (i.e., vacuoles shown in bright yellow spots) that facilitate embryo development in the absence of external energy sources–and that seem to give rise to the THG signal (see Fig. 3).2, 4, 6 This finding demonstrates that individual cells can be outlined and each nucleus can be identified.
Embryogenesis/morphogenesis processes can take several hours. During this time, THG microscopy at 1550 nm caused no apparent damage to the sample. In order to study the effect of the irradiation on the embryos, the team determined survival rates after long-term imaging and classified the embryos into two groups. Embryos irradiated before the two-fold stage showed a survival rate of 41% compared to an unexposed control group, while embryos that were irradiated after the two-fold stage were able to hatch normally and showed regular anatomical characteristics (survival rate of 73%). These findings demonstrate that the increased water absorption in tissues at IR wavelengths does not severely compromise sample viability. This is due to the fact that an efficient THG signal can be obtained at very low average powers (i.e. 4.9 mW) at the sample plane.
Multiple benefits
These experiments demonstrate the strong potential of THG at 1550 nm for high-resolution four-dimensional (4D) imaging of morphogenesis/embryogenesis in in-vivo samples. The technique seems to have potential to enable label-free cell tracking in early cell-division stages for cell linage studies. In contrast to other "label-free" microscopy techniques, such as coherent anti-Stokes Raman scattering (CARS), which offers a high chemical selectivity, THG is not selective to specific structures.8 However, its technical realization is very simple, as only a single laser is required.
Keeping in mind that the THG signal is sensitive to all interfaces, it is possible to outline its potential to enable label-free cell tracking in early cell-division stages and for cell lineage studies.
The FFS fiber laser simplifies the integration of non-linear microscopy techniques in biological research because it is compact, robust, inexpensive and requires minimal maintenance (see Fig. 4). Moreover, a frequency-doubled version of the same fiber laser source can be used for multimodal microscopy. In this way, it is possible to obtain THG data–along with that provided by other techniques such as two-photon fluorescence microscopy or SHG–in the same setup.
REFERENCES
- R. Aviles-Espinosa et al., J Biomed. Opt. 15 (4) (2010)
- W. Supatto et al., PNAS 102 (4): 1047-1052 (2005)
- S.W. Chu et al., Opt. Express 11 (23), 3093-3099 (2003)
- D.W. Debarre et al., Opt. Lett. 29 (104): 2881-2883 (2004)
- C.K. Sun et al., J. Struct Biol. 147 (1): 19-30 (2004)
- D. Debarre et al., Nat. Methods 3 (1): 47-53 (2006)
- E.J. Gualda et al., J. Microscopy 232 (2): 270-275 (2008)
- T. Hellerer et al., PNAS 104 (37): 14658-14663 (2007)
RODRIGO AVILES-ESPINOSAis a Ph.D. candidate, andPABLO LOZA-ALVAREZis Group Leader for ultrafast imaging and nonlinear microscopy at ICFO - The Institute of Photonic Sciences (Barcelona, Spain); www.icfo.es.WILHELM KAENDERSis President at Toptica Photonics AG (Graefelfing, Germany); www.toptica.com; [email protected]; ANDREAS BRODSCHELMis Scientist - R&D; [email protected].
More BioOptics World Current Issue Articles
More BioOptics World Archives Issue Articles