NEUROLOGY/IN-VIVO IMAGING: Doubling up for brain imaging
A high-performance, low-cost system that combines two optical approaches for simultaneous application in dynamic brain imaging promises exciting insight for preclinical, and eventually for clinical, work.
ByOfer Levi
In neuroimaging, the use of optical techniques for producing wide-field maps of hemodynamics (blood circulation) is increasingly prevalent. Optical equipment is attractive because it produces data with high spatial and temporal resolution, and is inexpensive compared with standard clinical techniques such as functional MRI (fMRI), SPECT, or PET that neuroscientists have come to rely on.
Two methods—intrinsic optical signal imaging (IOSI) and laser speckle contrast imaging (LSCI)—are often used as complementary methods for quantifying blood oxygenation and flow speeds, respectively.1 Simultaneous measurement of both oxygenation and flow rates provides more data than does fMRI, which generally cannot distinguish clearly between the effects of oxygen metabolization and compensatory flow.2 This distinction is important in providing a thorough understanding of the mechanisms of disease in scenarios such as stroke and epilepsy. In epilepsy, for example, researchers would like to know why increases in cerebral blow flow are seen prior to seizures, and whether this increase is sufficient to supply increased metabolic demand.3 In stroke, understanding flow as well as oxygenation changes is a key factor in determining the extent of the ischemic core and of the penumbra.4 Monitoring hemodynamics effectively after an ischemic event can also be used to measure and understand stroke recovery.5
So far, though, simultaneous measurement has been difficult because of the complexity of the required system. Recently, we demonstrated a novel implementation of combined IOSI and LSCI measurement using vertical-cavity surface-emitting laser (VCSEL) technology. The system design is progressing toward application to freely moving animals, and has important implications for clinical use with humans.6
Animal models and system considerations
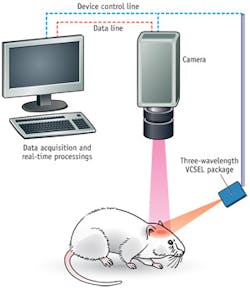
While animal models can provide insight into diseased states as they would apply to humans, inherent restrictions compromise the applicability of many imaging studies. General anesthesia is almost always necessary, and its effects on hemodynamic responses limit the neural signals that can be imaged.7 As a result, portable instrumentation and real-time techniques are necessary to allow imaging of fully conscious, active animals participating in normal behaviors.
Attempts to implement portable optical approaches in neuroimaging have produced some success. Portable two-photon techniques have provided high-resolution images of flow and anatomy, but are limited by the same factors that apply in large-scale two-photon imaging: Both the tabletop laser and the dyes needed are costly, and the laser requires alignment between sessions.8 Using fiber bundles to illuminate and collect fluorescent emission enables production of very high-resolution images, but reduces light collection efficiency and limits the field of view. For these reasons, fully integrated, wide-field imaging techniques are preferable in portable imaging.
Several portable systems based on modalities such as LSCI or fluorescence (and made feasible by small-scale CMOS imagers and compact illumination, or using tethered fiber-optics to bring illumination to and from an animal) have been introduced recently.9,10 While able to produce full-field imaging, the solutions are limited to single-modality applications. The inclusion and control of multiple illumination sources and optical components needed for multiple imaging techniques, operated simultaneously, leads to further complexities in the development of portable sensors.
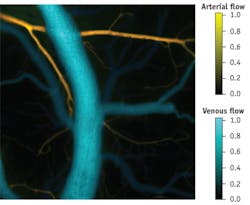
Our work has demonstrated a system that combines LCSI and IOSI simultaneously, using compact laser sources, to monitor cortical ischemia in animals, in a full-field format with high temporal acquisition rates (more than 15 integrated frames per second). The combined measurements of speckle contrast and oxygenation enabled us to establish absolute flow velocities, and to statistically distinguish between veins and arteries (see Fig. 1).
We built the system using coherence-reduction techniques applied to VCSELs operating at 680, 795, and 850 nm, and a straightforward optical design. The result is a setup that can be easily adapted into a portable format for continuous monitoring (see Fig 2).
Why VCSELs?
For the past 20 years, scientists have relied on high-end back-illuminated optical imaging to measure oxygenation dynamics. This type of experiment requires a light source that behaves like a very quiet lamp; lasers are typically too noisy due to speckle-related intensity fluctuations, which are common for coherent light sources. But creating a map for brain blood flow does require a coherent laser—so conducting both measurements requires an additional setup.
Thanks to their thermal properties, VCSELs show unique potential in their application to portable imaging. They can be used to produce coherent and incoherent illumination in a rapidly alternated scheme, allowing rapid, dual-modality imaging using a single, low-power illumination source. Injecting current into the laser rapidly changes properties, allowing the laser to move between high- and low-coherence modes. This approach enables measurement of two modalities with a single system—which enables lower costs and co-registration of imagery. In addition, dual modality allows us to infer from one measurement technique to the other to calibrate flow speeds, as well as to rapidly classify arteries and veins.
While LEDs still generally provide lower noise than current swept VCSELs, in the scheme described here VCSEL noise can be brought within the shot noise limit, eliminating any apparent performance differences. A compact VCSEL source further allows for rapidly pulsed imaging that is not generally feasible with other laser sources. Considering the benefits discussed here, the use of VCSELs can lead to a system which is simplified in cost, power consumption, and complexity, and thus represents an important step towards developing a portable imaging system.
We chose to work within the near-infrared (NIR) wavelength region, where light penetrates deeply into tissue. We selected the three VCSEL wavelengths based on available technologies, and matched them to three regions of the Hb spectra that provide important signals for IOSI: 680 nm is near a minimum of hemoglobin oxygenation absorption, and reflects mostly changes in deoxygenation; 795 nm is near an isobestic point of the spectra, and reflects total hemoglobin changes; and 850 nm is near a minimum for deoxygenation absorption and reflects mostly oxygenation changes. We characterized the wavelengths, and used the resulting data to estimate the expected contrast reduction in tissue.
Camera integration
We chose the EMCCD image sensor as a proof-of-concept for the VCSEL technique, to provide rapid imaging with high sensitivity and low noise: Our setup uses a Rolera EM-C2 14-bit camera by QImaging (Surrey, BC, Canada) to image the cortex illuminated by VCSELs. The system has been tested with wide-field imaging lenses producing magnifications of 1 to 4x. The maximum field of view is 1004 × 1002 pixels, each 8 × 8 μm.
The Rolera camera provides low read noise, allowing us to more rapidly detect low light signals and high sensitivity, which enabled us to take advantage of extremely high frame rates. We were able to show dynamics of both flow and oxygenation changes in response to induced ischemia in a wide field of view, with speeds that at times exceeded 100 fps, making it possible to calibrate the entire brain flow map.
Results of our tests indicate that our rapidly alternating VCSEL operation allows for very high quality, high temporal acquisition imaging of flow and oxygenation. The noise levels are sufficiently low to achieve sensitivity to micromolar concentration changes, and flow changes on the order of 10% or lower. The noise observed in IOSI is largely limited by shot noise, and further averaging can be used at this high acquisition rate to observe signal changes well below 0.1% at each wavelength. Signals at this level are commonly seen in sensorimotor stimulation studies.11, 12 Thus, the method is demonstrated to be useful for simultaneous imaging of cerebral blood flow and oxygenation using a compact light source. We have been able to track wide area dynamics rapidly, and assert that this technique can be applied to long-term monitoring.
We see this method having important applications in the study of stroke progression and recovery, as well as in evaluation of drugs and treatment for brain and disease therapy. In particular, we see further development in miniaturizing this technique to produce a portable device. With portable and continuous monitoring of hemodynamics, we can gain a better understanding of stroke conditions such as periinfarct depolarizations (PIDs) and how they relate to flow changes in the ischemic penumbra.13,14 Understanding the relation between flow and metabolization in the ischemic core and penumbra is a key factor in determining the mechanisms of cell death during stroke.15 In epilepsy, flow and oxygenation information can be used to understand the temporal precedence of metabolic changes.3,16,17
High temporal and spatial resolution and wide-field imaging are very important aspects of obtaining a full picture of neural activity. For future studies, we envision the addition of fluorescence imaging to improve the versatility of available techniques. Calcium-sensitive dyes can be used to directly measure ions associated with neural activity in epilepsy. Alternatively, potassium-sensitive dyes can be applied alongside LSCI to understand the effect of ion transfer or spatial buffering in glial cells during seizures.18-20 Correct emission filter and laser wavelength selection is required to allow proper blocking of excitation wavelength, while passing the wavelengths needed for LSCI and IOSI.
Continuing progress
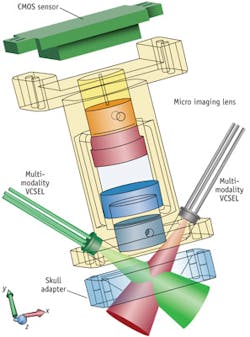
For the future, we aim to create a smaller, multi-modality version of the system that will allow the animal to move freely during the experiments (see Fig. 3). This will enable long-term chronic monitoring of epilepsy dynamics and stroke recovery in preclinical studies. Longer-term, we expect the approach will give neuroscientists the ability to better understand the brain dynamics for patients who have suffered from epileptic attack, stroke, or traumatic brain injury (TBI). Currently, it takes 6–12 weeks to measure brain activity after a treatment is given. With our research, we hope to provide real-time feedback on the effect of a treatment by detecting changes in the brain within a day instead of weeks. This rapid feedback should also improve research and development efforts for personalized medicine.
Our system may someday enable researchers to pinpoint metabolic changes in the brain that occur just moments prior to an epileptic seizure, or may help doctors map the brain's "areas of eloquence," those areas that need to remain untouched during surgery on epileptic patients. Currently these areas are mapped electrically, over sometimes extended periods of time and with great discomfort to the patient.
Other clinical applications may include helping researchers create brain-interface technology that would allow researchers "to decode [disabled children's] intentions in the absence of speech and gestures," according to Dr. Tom Chau, associate professor at IBBME and senior scientist at the Holland Bloorview Kids Rehabilitation Hospital.
REFERENCES
1. P. B. Jones et al, J. Biomed. Opt., 13, 044007 (2008).
2. F. Di Salle et al., European J. of Radiol., 30, 84–94 (1999).
3. T. H. Schwartz, S.-B. Hong, A. P. Bagshaw, P. Chauvel, and C.-G. Benar, Epilepsy Res., 97, 252–266 (2011).
4. A. K. Dunn, H. Bolay, M. A. Moskowitz, and D. A. Boas, J. Cereb. Blood Flow Metab., 21, 195–201 (2001).
5. J. C. Eliassen et al., Top Stroke Rehabil., 15, 427–450 (2008).
6. H. Levy, D. Ringuette, and O. Levi, Biomed. Opt. Exp., 3, 777–791 (2012).
7. K. Masamoto, T. Kim, M. Fukuda, P. Wang, and S.-G. Kim, Cereb. Cortex, 17, 942–950 (2007).
8. B. A. Flusberg et al., Nat. Meth., 2, 941–950 (2005).
9. P. Miao, H. Y. Lu, Q. Liu, Y. Li, and S. B. Tong, J. Biomed. Opt., 16, 3 (2011).
10. K. K. Ghosh et al., Nat. Meth., 8, 871-U147 (2011).
11. C. H. Chen-Bee, T. Agoncillo, Y. Xiong, and R. D. Frostig, J. Neurosci., 27, 4572–4586 (2007).
12. A. J. Blood, and A. W. Toga, J. Cereb. Blood Flow Metab., 18, 968–977 (1998).
13. H. K. Shin et al., J. Cereb. Blood Flow Metab., 26, 1018–1030 (2006).
14. K. A. Hossmann, Cerebrovasc. Brain Metab. Rev., 8, 195–208 (1996).
15. D. A. Boas, and A. K. Dunn, J. Biomed. Opt., 15, 011109 (2010).
16. M. R. Zhao et al., J. Neurosci., 31, 13292–13300 (2011).
17. T. H. Schwartz, Epil. Curr., 7, 91–94 (2007).
18. P. Padmawar, X. Yao, O. Bloch, G. T. Manley, and A. S. Verkman, Nat. Meth., 2, 825–827 (2005).
19. F. Amzica, M. Massimini, and A. Manfridi, J. Neurosci., 22, 1042–1053 (2002).
20. S. Dufour, P. Dufour, O. Chever, R. Vallee, and F. Amzica, J. Neurosci. Meth., 194, 206–217 (2011).
Ofer Levi is Assistant Professor at the University of Toronto's Institute of Biomaterials and Biomedical Engineering (Toronto, ON, Canada; www.ibbme.utoronto.ca) and lab director for the biophotonics group there. Contact him at [email protected].