MOLECULAR IMAGING/SURGICAL GUIDANCE: NIR image-guided surgery: Progress and needs for clinical application
MERLIJN HUTTEMAN
Despite recent advances in cancer treatment, surgical removal remains the most important therapy for solid tumors. Cancer surgeons walk a fine line between making sure they remove all the diseased tissue and minimizing damage to structures such as nerves, bile ducts, and ureters that are often compromised during operations. While a number of imaging technologies exist to help surgeons visualize tumor size, location, and proximity to vital structures preoperatively; once in the operating room, surgeons make decisions mainly based on the error-prone techniques of palpation and unaided visualization.1 As a result, insufficient resection and damage to vital structures occur relatively frequently.2-4
Optical imaging using near-infrared (NIR) fluorescence has potential to provide real-time information during surgery on the exact location of tissues to be resected and those to be avoided. The approach has the potential to be of major impact on surgical practice generally—and on cancer surgery in particular.1 It promises to improve outcomes and reduce complications in a wide variety of procedures. Since NIR fluorescence imaging involves no ionizing radiation, requires only relatively low-cost camera systems, and can be used for real-time visualization during surgery, the technique could be used around the world, including areas with limited healthcare budget.
Advantages of NIR fluorescent light (700–900 nm) include high tissue penetration (millimeters to centimeters deep) and low autofluorescence, thereby providing sufficient contrast.5 Because the human eye is insensitive to NIR wavelengths, the use of NIR light does not alter the surgical field.
The state of the art
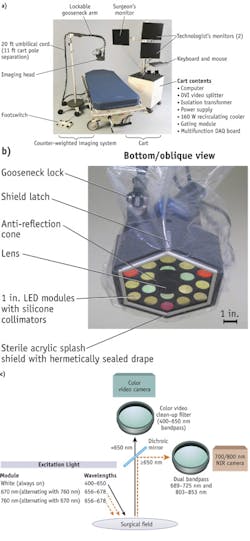
Recently developed intraoperative NIR systems are able to provide simultaneous acquisition of surgical anatomy (white light, color video) and NIR fluorescence signal.6-8 And, as evidenced by Frangioni's Fluorescence-Assisted Resection and Exploration (FLARE), systems are available that can simultaneously acquire and display multiple separate fluorescence wavelengths, enabling labeling of tumors on one channel and vital structures on a second channel (see Fig. 1). Therefore, the use of NIR fluorescence could potentially be of great value in the intraoperative detection of critical anatomical structures and oncologic targets.
The other ingredient key to NIR fluorescence-guided surgery is exogenous contrast agents for visualizing specific tissues. Currently, the only NIR fluorescent contrast agents registered by the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) for clinical applications are the indocyanine green (ICG; peak emission ≈ 820 nm) and methylene blue (MB; peak emission ≈700 nm), neither of which is able to target tumor cells. Clearly, there is need for more.
My recent doctoral thesis, "Image-guided surgery using invisible near-infrared fluorescent light: from pre-clinical studies to clinical validation," explores in depth the topic of NIR-guided surgery, and highlights work done at Leiden University Medical Center (LUMC; The Netherlands). It summarizes the state of the art and details research performed under supervision of Alexander Vahrmeijer and Prof. Cornelis van de Velde, both surgeons at LUMC, in close collaboration with John Frangioni, a professor of Medicine at Harvard Medical School (Cambridge, MA). This article is drawn from that publication, which is available at the Leiden University website: https://openaccess.leidenuniv.nl/ handle/1887/17805.
Sentinel lymph node detection
Sentinel lymph node (SLN) mapping, as introduced in the treatment of cutaneous melanoma by Donald Morton, is now considered part of the standard of care in cutaneous melanoma, breast cancer, and vulvar cancer.9 The SLN is the lymph node that drains directly from the tumor and is therefore most likely to be the node to which tumor cells will first metastasize. By injecting a tracer around the primary tumor, the SLN can be identified and resected by following the drainage of that tracer. If the SLN contains no tumor cells, it is unlikely that the remaining lymph nodes contain metastases and the resection of these nodes and the associated comorbidity can be avoided.
Currently, SLN mapping typically involves the injection of a radiotracer preoperatively and injection of a blue dye shortly prior to surgery. Using this combined technique, high sensitivity and low false negative rates are achieved. However, these methods have their disadvantages. The use of a radiotracer exposes caregivers and patients to ionizing radiation and may not be possible in all clinics due to regulatory issues. Local injection of a blue dye stains the surgical field in an unnatural color that persists for several months after surgery and cannot be visualized when it is covered by overlying tissue.
The use of NIR fluorescence has the potential to overcome these limitations and simplify the use of SLN mapping in various cancer types. Indeed, the first studies have been described that use the currently available ICG in combination with an intraoperative NIR fluorescence imaging system for SLN mapping in breast cancer, gynecologic cancer, and gastrointestinal malignancies.6,7,10-12
Tumor margin detection and image-guided resection
Different strategies can be followed to detect malignant cells or tissues during surgery. The various hallmarks of cancer can be used as a target for imaging strategies: Increased growth and growth factor signaling receptors, limitless replicative potential, sustained angiogenesis, and increased proteolytic activity resulting in tissue invasion and metastasis.13 Development of probes for these purposes is the focus of intensive research, and many have been tested in preclinical settings. Enzyme-activatable probes allow detection of proteases that are relatively abundant in malignant tissue, which can be associated with specific characteristics of the tumor; for instance, invasive, aggressive, or metastatic tendency. These agents are injected in a quenched (i.e., non-fluorescent) state, resulting in minimal fluorescence at the time of administration. After cleavage by the specific enzyme, the agent becomes dequenched (i.e., fluorescent), resulting in a high signal-to-background (SBR). Examples of activatable agents that have been used in animal cancer models are activatable polyarginine-based cell-penetrating peptides that detect matrix metalloproteinases, activity-based probes that target cysteine cathepsins, and several probes developed by Ralph Weissleder and colleagues at Harvard Medical School and Massachusetts General Hospital, which are activated by cathepsins or matrix metalloproteinases and are now available commercially (through PerkinElmer; Waltham, MA).14-17
Rather than detecting tumor-associated proteases, cancer cells can be detected with molecular specificity using a targeting ligand or monoclonal antibody conjugated to a fluorophore. Tumor detection by exploiting the increased growth factor receptor expression of tumors has been described in all kinds of different tumors. In these studies, fluorophores that were coupled to monoclonal antibodies targeting the epidermal growth factor receptor, Her2/neu receptor, or vascular endothelial growth factor receptor were used.18-21 For imaging of tumor angiogenesis, targeting of alpha-v-beta-3 (αvβ3) integrin, a critically important adhesion molecule in the regulation of angiogenesis, is a widely used strategy. Targeting of αvβ3 integrin by cyclic arginine-glycine-aspartate conjugated to various non-quenched or quenched fluorophores has been reported.22-24 Finally, in analogy with positron emission tomography (PET) technology, increased glucose metabolism due to increased expression of membrane glucose transporter proteins in intracranial gliomas has been reported.25
Identifying vital structures
In order to identify vital structures by using NIR fluorescence contrast agents, a distinction should be made between hollow and solid structures. Hollow structures, such as bile ducts, ureters, and blood vessels, can be visualized if contrast agents can be delivered intraluminally, whereas solid structures like nerves can be visualized by targeting specific cellular markers as membrane proteins.
Hollow structures can be visualized either by direct injection or by means of excretion from, for example, the liver or kidneys. ICG is excreted by the liver into the bile and has been shown to identify bile ducts during surgery.26-28 MB is cleared both hepatically and renally, and has been shown to identify both ureters and bile ducts.29
Identification of nerves requires the development of novel contrast agents. Advances have been made in the development of nerve targeting probes, although many hurdles still exist as the probes are fluorescent in a lower wavelength than the NIR spectrum, thereby lowering tissue penetration and suffering from increased autofluorescence.30 Another strategy is to use fluorescent peptides that target the nerve sheath, avoiding the need to cross the blood-nerve-barrier.31
Preclinical validation
NIR fluorescence image-guided surgery has been validated preclinically, and work is underway to translate the technology and technique to clinical applications.
In terms of preclinical work, rat tumor models provided a platform for much discovery. For instance, we were able to demonstrate intraoperative identification of colorectal liver metastases using NIR fluorescence and a novel integrin αvβ3 targeted probe. In this study, an NIR system was able to identify all of the induced colorectal liver metastases as well as several intra-abdominal metastases.32
Another rat model study, focused on breast cancer tumor margins, was inspired by the fact that positive resection margins are a major problem in the treatment of breast cancer, with reported rates of up to 40 percent. A protease-activatable NIR fluorescent probe has proven helpful for intraoperative identification of tumor margins of breast cancer. Subsequently, tumors were resected under direct image-guidance, with minimal excision of healthy tissue.33
Finally, a study focused on optimization of the clinically available probe indocyanine green (ICG) used a syngeneic rat model. ICG, which is cleared by the liver, has been shown to passively accumulate around colorectal liver metastases. The work demonstrated intraoperative identification of all metastases following ICG injection.34
Clinical translation
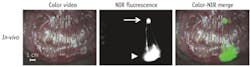
Other work has focused on clinical application. SLN analysis is important in the staging and treatment of breast, cutaneous melanoma, and vulvar cancers; while the procedure has been studied extensively in colorectal cancer patients, it has yet to show clear benefit—which could be the result of suboptimal technique. As an oncologic resection involves the en bloc resection of regional lymph nodes, ex-vivo tracer injection and SLN mapping are possible. We studied the use of NIR fluorescence imaging for ex-vivo SLN mapping in combination with an experimental, more optimized probe in colorectal cancer patients (see Fig. 2). The technique was optimized in a swine model and subsequently tested in a pilot clinical trial. In all cases, NIR fluorescence imaging enabled the detection of SLNs. In one case, a mesenteric metastasis was encountered that was not NIR-fluorescent; however, this was a tumor mass without any remaining lymph node tissue, preventing lymphatic flow.35SLN mapping in breast cancer typically involves use of a radiotracer and blue dye. NIR fluorescence imaging has the potential to improve SLN mapping in breast cancer by replacing one of the current modalities, or even both, or functioning as an adjunct. In a study exploring imaging system optimization and ICG injection, ICG was premixed with human serum albumin (HSA) to increase retention in the SLN and increase fluorescence brightness of ICG. SLN mapping using NIR fluorescence was uneventful in all patients and allowed detection of 1.45 SLNs on average. Optimal ICG:HSA dose was 400 to 800 μM.36
In earlier preclinical studies, premixing of ICG with HSA showed clear advantages: It improved the retention of the dye in the SLN and increased the fluorescence brightness.37 One study aimed to test this advantage in a clinical, randomized setting, as injection into a human breast might induce coupling with the physiologically available albumin and eliminate the need of premixing. Patient groups injected with or without premixed ICG showed no difference in fluorescence contrast (P = 0.18), or in number of identified nodes (P = 0.74), indicating that premixing of ICG with HSA can be omitted in case of breast cancer. This simplifies the procedure and can facilitate the introduction of this technique in clinical practice.38
In work done to study the use of NIR fluorescence for the intraoperative detection of SLNs in cervical cancer, patients received peritumoral injections of ICG:HSA shortly before surgery. After exposure of lymph node basins, NIR fluorescence imaging enabled successful detection of SLNs in all patients. No false negatives were observed.39 Another study explored the use of NIR fluorescence in SLN mapping in vulvar cancer patients, who also received standard-of-care injections of radiotracer and patent blue dye. In all patients, SLNs (N = 11) could be detected by NIR fluorescence and radiotracer; three nodes, however, were not blue. These pilot studies show the successful use of NIR fluorescence imaging in the detection of SLNs in gynecologic malignancies.40
Nowadays, the majority of breast cancer resections are breast-conserving surgeries, where only the tumor and a safety margin around it are resected. When a mastectomy is performed, several reconstructive techniques are available. The use of free skin flaps is associated with good cosmetic results and high patient satisfaction. The surgical procedure, however, can be challenging and creation of skin flaps for autotransplantation requires careful planning to select the right blood vessels for optimal flap perfusion. We assessed the use of NIR fluorescence for visualizing flap vascularization in a clinical trial of patients undergoing deep inferior epigastric perforator flap reconstruction after mastectomy. ICG was injected at three dose levels and NIR fluorescent angiography was performed at fixed moments during surgery. NIR fluorescence permitted visualization of flap vascularization in all patients.41
Intraoperative visualization of pancreatic tumors could help reduce the number of irradical resections. As no tumor-specific probes are clinically available, we conducted a study that involved injecting ICG in order to test if tumors could be identified by passive accumulation (the enhanced permeability and retention effect). Furthermore, as ICG is excreted into bile, it was evaluated if bile ducts could be visualized intraoperatively. Unfortunately, no useful tumor contrast could be observed in all but one patient. NIR fluorescence did, however, enable the identification of extrahepatic bile ducts during surgery.42
ICG injection was the basis for another study, too, in which patients with colorectal liver metastases received the probe prior to surgery. During surgery, superficially located metastases could clearly be identified by a fluorescent rim around the tumor. This could be caused by hampered excretion of ICG into bile, by compression of liver tissue by the expanding tumor. Importantly, other than tumors identified preoperatively by CT or MRI, and intraoperatively by visual inspection and palpation, NIR fluorescence enabled the detection of four hotspots not found by other modalities and histologically confirmed to be metastases. Tumor-to-liver ratios of 7.4 (range of 1.9–18.7) were observed, which is higher than any of the preclinically tested, tumor-targeted probes.43
Probe futures
While ICG and MB were not designed as contrast agents for image-guided surgery and are not optimal (for instance, they cannot be conjugated to targeting ligands), the availability of clinically approved NIR fluorescent probes has been essential for the first clinical trials. But for NIR fluorescence imaging to perform up to its full potential and have a significant impact on patient care, several new developments are necessary.
Tumor- and nerve-specific probes need to be approved for clinical application.30, 31 IRDye 800CW (LI-COR Biosciences; Lincoln, NE), for instance, matches these requirements and has recently completed its toxicity tests in rodents.44 Choi et al. have shown that quantum dots can be cleared rapidly from the body, if the hydrodynamic diameter is smaller than 5.5 nm and the surface charge is balanced of the molecule.45 Following these observations, the Frangioni Lab at Harvard Medical School has developed a novel organic fluorophore that is zwitterionic (ZW800-1).46 Both IRDye 800CW and ZW800-1 are manufactured following cGMP guidelines, and it is expected that clinical studies can start within the next months to years. Future research should be focused on maximizing the fluorescent properties of probes, optimizing rapid excretion, and further reduction background uptake.47
To selectively label tumor cells, various distinguishing hallmarks of cancer can be used as targets.48 An optimal target is exclusively and abundantly expressed by tumor cells and can be targeted without causing toxicity. Novel NIR fluorescent probes have been developed that target growth factor receptors, glucose metabolism, angiogenesis, and enzymatic activity, and these probes have been studied in preclinical tumor models.14, 18-24, 49 First-in-human results of intraoperative fluorescence imaging in debulking surgery for metastatic ovarian cancer have been reported with a folate-receptor targeted probe.50 Although the probe used in these studies was based on fluorescein, which fluoresces in the non-optimal visible light spectrum, these results are highly promising for clinical application of targeted NIR fluorescent probes in image-guided surgery.
Iatrogenic nerve damage is a major complication in oncologic surgery, which could potentially be avoided by NIR fluorescence imaging. Nerve-specific agents studied to date have significant limitations.30, 31 Future research will have to show what strategy is most optimal to selectively target nerves.
Systems design
Each imaging system currently available has drawbacks: Some show NIR fluorescence signal without displaying anatomical context, others are relatively large, and most are not sufficiently user-friendly.6,7, 51, 52 Furthermore, laparoscopic systems are not widely available, and current systems do not provide anatomical context.28 Depth penetration of NIR light is limited and novel camera designs are increasing the depth at which a fluorophore can be detected.
Various strategies can be followed to achieve greater depth. Detection of tissue autofluorescence will minimize background noise and increase the depth at which a fluorophore can be detected. For this purpose, fluorescence lifetime imaging (FLIM, which measures the decay of fluorescence intensity of a fluorophore) can be utilized.53 Temporal and spatial frequency domain modulation of the light source can be used to determine depth information of the fluorescent signal.54 Optimized camera systems are being developed by various groups and companies, and research is focused on improving performance and the ease of use in the operating room.
When these optimized imaging systems become available, NIR fluorescence imaging has a chance to leap from the research setting into general clinical practice.
REFERENCES
For the list of references, please visit www.laserfocusworld.com/home/article/14193612/references.
Merlijn Hutteman, Ph.D., is a medical student in the Department of Surgery at Leiden University Medical Center in Leiden, The Netherlands (www.lumc.nl). Contact him at [email protected].