TWO-PHOTON MICROSCOPY/MULTIMODAL IMAGING: Femtosecond laser developments advance two-photon imaging
Growth in multiphoton microscopy has been driven by advances in laser technology—advances that have also facilitated access to multimodal imaging. For instance, a femtosecond laser that enables multiphoton imaging of multiple features in a specimen simultaneously over a wide range can also enable second-harmonic generation (SHG) and even coherent anti-Stokes Raman scattering (CARS) microscopy.
ByPhilip G. Smith, Tommaso Baldacchini, John Carter, and Ruben Zadoyan
Multiphoton microscopy (also known by such names as multiphoton excitation fluorescence microscopy, two-photon laser scanning microscopy, and nonlinear microscopy), a technique valued for its ability to penetrate deep into living tissue, has experienced rapid growth recently. This growth has been driven in part by advances in the laser technology that is at the heart of the technique: Major developments in femtosecond lasers impacting this application include:
1. Availability of wavelengths into the infrared range. For most specimens, the scattering cross section (the area that describes the likelihood of light scattering) decreases as wavelengths increase.1,2 Thus, ballistic photons in the upper spectrum of the relative transparency window (that is, the infrared range, typically between 650 and 1300 nm) can penetrate deeper into the specimen.
2. Short pulse widths. Because the multiphoton excitation process is actually quite inefficient, peak power is important for a femtosecond source in this application. And because peak power is inversely proportional to pulse width, it is beneficial to have the shortest pulse width to provide the greatest possible fluorescence generation.
3. Dispersion compensation. When femtosecond pulses travel through optical materials such as those found in standard commercial microscopes, the pulses are temporally broadened due to dispersion. Pre-compensating the pulses by adding what is called negative dispersion can counteract this effect to produce brighter images with higher contrast.
4. Dual wavelengths. Many confocal microscopes use multiple inexpensive continuous-wave lasers, each generating a different wavelength. An advantage of this approach is the ability to image two or more fluorophores simultaneously. The multiphoton technique necessitates the use of light sources that are tunable over a wide range of wavelengths, but to image more than one fluorophore simultaneously, it becomes quite expensive to have more than one laser system. For this reason, a single, tunable system able to produce two wavelengths is very valuable.
5. Simple, automated operation. Providing advanced capabilities in a simple, easy-to-use platform enables researchers to focus on their research instead of worrying about their equipment.
These technological advances have also facilitated access to multimodal imaging—which has proven to enable a better understanding of complicated biological processes.3 A femtosecond laser that enables multiphoton imaging of structures labeled with fluorescent proteins can also enable second harmonic generation (SHG) imaging to view ordered structures, such as collagen tissue.4,5 And if the same system includes a second pulse train that is synchronous with the main pulse train, it is possible to perform label-free imaging such as coherent anti-Stokes Raman scattering (CARS) microscopy to view structures such as lipids (see sidebar).6,7 Let's take a look at some of the imaging possibilities enabled by such a laser, in combination with commercially available multiphoton microscopy systems.
Multiple features over broad wavelength range
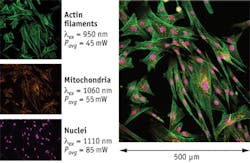
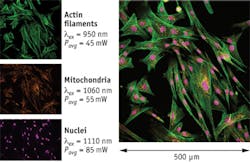
Figure 1 demonstrates the ability of a next-generation laser to image several different features of the same tissue over a wide wavelength range. Doing this allows researchers to determine how these features interact in a complex way that would be difficult to realize from a single image. The figure shows a multiple fluorescence image of muntjac skin fibroblast cells excited by two-photon absorption. The specimen was stained with Alexa Fluor 488 (actin filaments, green), Alexa Fluor 555 (mitochondria, orange), and TO-PRO-3 (nuclei, purple), all by Life Technologies (Carlsbad, CA). In order to produce the highest fluorescence intensity from each dye, the laser was tuned to 950, 1060, and 1110 nm wavelengths for Alexa Fluor 488, Alexa Fluor 555, and TO-PRO-3, respectively. The main image on the right was then obtained by stitching the three separate images together.
Multimodal imaging: SHG and CARS
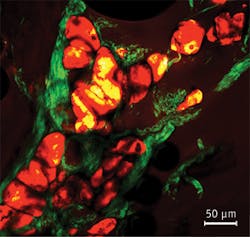
Figure 2, an example of multimodal imaging, shows another outcome made possible by a laser able to image multiple features simultaneously. The specimen was an unstained section of bovine muscle. The SHG signal from collagen fibers was recorded by using an excitation wavelength of 800 nm, while CARS signal from lipids within adipocytes was recorded by probing the CH2 symmetric stretching mode that is centered at around 2890 cm-1. This was achieved by employing pump and Stokes wavelengths of 800 and 1041 nm, respectively. An external delay line was used to add approximately 30 cm of optical delay to one of the two pulse trains to allow perfect temporal overlap at the sample plane. The two signals (SHG and CARS) were then recorded simultaneously.
The technique of CARS imaging has been used for many years with picosecond lasers, which feature narrower bandwidth than femtosecond lasers and therefore take full advantage of the chemical selectivity of the CARS imaging technique.8 However, these picosecond systems are not as useful for the standard two-photon-excited fluorescence and SHG imaging techniques which require high peak power. By combining all of these techniques in a single image, it is possible to gain significant information about the interaction of these various structures that might not be possible otherwise.
Chemical specificity over broad bandwidth
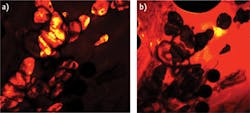
Figure 3 shows CARS images with chemical specificity taken from the same section within the specimen (bovine muscle) as Figure 2. Because the CARS imaging technique is based on vibrational energy contrast, it can differentiate between areas that have dissimilar chemical compositions. With the laser tuned to perform femtosecond CARS microscopy at 2890 cm-1, lipid-rich regions of the tissue produce a strong signal. With the laser tuned to perform CARS microscopy at 3200 cm-1, the image is completely reversed: Areas rich in lipids are dark, while the surrounding tissue (rich in water) produced a strong signal.
While the ability to achieve chemical specificity has long been a feature of continuous wave and picosecond CARS, this data demonstrates chemical specificity using the femtosecond CARS technique, proving that the broad spectral bandwidth of a femtosecond pulse does not inhibit observation of chemical selectivity for species such as lipids.
REFERENCES
1. Data obtained from http://omlc.ogi.edu/spectra/ and associated links.
2. K. Svoboda and S.M. Block, Annu. Rev. Biophys. Biomol. Struct., 23, 247–85 (1994).
3. S. Yue et al., Laser Photon. Rev., 5, 496–512 (2011).
4. P.J. Campagnola and L.M. Loew, Nat. Biotechnol., 21, 1356–1360 (2003).
5. Y. Guo et al., PNAS, 10854–10856 (1999).
6. A. Zumbusch, G.R. Holtom, and X.S. Xie., Phys. Rev. Lett., 82, 4142–4145 (1999).
7. J-X Cheng, App. Spectrosc., 61, 9, 197A (2007).
8. M.D. Duncan, J. Reintjes, and T.J. Manuccia, Opt. Lett., 7, 8, 350–352 (1982).
9. D. Kobat et al., Opt. Exp., 17, 13354–13364 (2009).
10. D. Kobat, N.G. Horton, and C. Xu, J. Biomed. Opt., 16, 10, 106014 (2011).
Philip G. Smith, Ph.D., is senior product marketing manager, Tommaso Baldacchini, Ph.D., is staff scientist, and John Carter is senior test engineer at Spectra-Physics, a Newport Corp. brand (Santa Clara, CA). Ruben Zadoyan, Ph.D., is senior director of advanced product development at Newport Corp.'s Technology and Applications Center (Irvine, CA). Contact Dr. Smith at [email protected].
A next-generation platform
Recently, researchers at Cornell University have shown that by going to a longer excitation wavelength (1275 nm) it is possible to image in-vivo up to 1.5 mm deep into brain tissue (compared to around 0.5 mm deep at a wavelength of 775 nm).9,10 In an effort to enable broad access to such capability, some researchers have explored longer wavelengths using Ti:sapphire pumped OPO solutions, but the cost and complexity of this approach has limited its use.9 Newport has introduced its next-generation laser, the InSight DeepSee, to address just this issue. The system combines the widest commercially available tuning range (680 to 1300 nm), a short pulse width (100 fs), and integrated dispersion compensation all in a single package that has been designed for ease of use. The practical advantages of these features mean that the end user can seamlessly scan over the full tuning range, with the beam exiting a single output port. The combination of a short pulse width and integrated dispersion compensation results in higher brightness, better contrast, and deeper penetration.
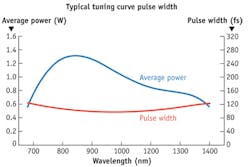
For most imaging applications, the average power is reduced to below 50 mW in order to avoid such problems as photodamage (where tissue is actually damaged) and photobleaching (where the fluorescent molecules generating the image no longer fluoresce). But this is not necessary with the InSight DeepSee. In Figure 4, the blue curve (which references the left-hand y-axis) shows the typical average power produced by the system while the red curve (which references the right-hand y-axis) shows the typical pulse width as a function of output wavelength. The pulses are consistently short (85–120 fs) over the entire tuning range, whereas most Ti:sapphire-pumped OPO solutions deliver longer pulses (200 fs or more) in the 1000–1300 nm range. So, with half the pulse width, one can achieve twice the fluorescence signal for the same average power.
As an option, the system can be configured with a second output, delivering femtosecond pulses at 1041 nm with approximately 500 mW of average power. Coupled with the primary beam covering 680–1300 nm, this secondary beam supports dual-wavelength imaging, where two features of tissue can be imaged simultaneously (see Fig. 1). Examples include using 1041 nm plus 930 nm for simultaneous imaging of mCherry and GFP, respectively, or using 1041 nm for mCherry, while also imaging using THG at 1200 nm. Since both pulse trains are also temporally synchronized, they can be combined in a femtosecond CARS imaging setup to image lipids via the C-H stretch vibrations at about 2890 cm-1, for instance using 1041 nm as the Stokes wavelength and 800 nm as the pump wavelength.
More BioOptics World Current Issue Articles
More BioOptics World Archives Issue Articles