OPTICAL MOLECULAR IMAGING/SURGICAL GUIDANCE: 'Painting' tumors to guide cancer surgery
Alexandra De Lille
While oncologists have access to an array of therapies including radiation, chemotherapy, and biological therapeutics, surgical removal of tumor tissue remains a primary strategy. Imaging modalities such as MRI, PET, SPECT, CT, and ultrasound enable noninvasive imaging and detection of cancerous tissue, but these techniques do not readily highlight diseased tissue during surgery. So the surgeon must rely on experience and mentally integrate preoperative images with palpable or visual indication of the tumor boundary.
Fluorescent highlighting of diseased tissue, potentially complemented with multicolor labeling of vascularization and innervation (all visualized in real time during surgery), promises to provide an additional "set of eyes" for the surgeon. This approach would improve the margin of resection and consequently provide a better prognosis. Furthermore, it would increase the accuracy of intrasurgical disease staging and speed the surgical process—all without involving radioisotopes.
Tumor Paint
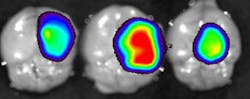
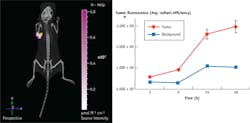
This is the concept behind Tumor Paint, a technology introduced by James M. Olson, MD, Ph.D., and colleagues of Seattle Children's Hospital and The Hutchison Cancer Center. It involves use of a targeted fluorescent probe that seeks and specifically binds tumors, with the promise to facilitate intraoperative visual discrimination of healthy vs. diseased tissue. The approach promises particular benefit for brain tumor resection, where the accidental removal of healthy tissue directly impacts the patient's functionality upon recovery—and on the other hand, incomplete tumor resection augments relapse.Currently, this approach is being trialed at the veterinary research level with Blaze Bioscience, a startup company founded by Dr. Olson. Translational studies at the Washington State University Veterinary Teaching Hospital have used PerkinElmer's beta Open Air Fluorescence Imaging System (see Frontis) to visualize solid tumors in canine patients with Tumor Paint.
Clinical adaptation of tumor-targeting molecules
The concept of creating tumor targeting molecules has been pursued extensively with the development of molecular imaging. As opposed to anatomical imaging where one defines disease by changes in anatomy (with, for example x-ray, CT, MRI, or ultrasound), molecular imaging uses probes or reporter genes to identify the biology of the disease. In clinics, this is mostly accomplished with PET or SPECT and radionucleotide-tagged probes such as 18F-FDG, a glucose analog that identifies tumors based on the Warburg effect that is characterized by a higher rate of glycolysis than normal cells.
Traditionally, clinical imaging modalities are adopted in the preclinical environment. Optical molecular imaging originated almost two decades ago in the preclinical arena because of easy access, relative affordability, lack of radiosisotope requirement, versatile application platform, and high sensitivity. Now, we are observing adaptation in the clinic—not only for tumor detection, but also for the imaging of inflammation, infection, angiography, and transplantation. A significant limitation is the fact that only one NIR fluorophore—namely Indocyanine Green (ICG)—is approved for clinical use. ICG imaging is currently applied clinically for sentinel lymph node mapping, retinoscopy, angiography, hepatobiliary cancer, perivascular disease and, to a certain extent, for tumor imaging based on the enhanced perfusion and retention (EPR) effect. Unfortunately, this compound lacks a conjugation group, prohibiting the generation of tumor-targeting moieties that increase specificity.
Great progress has been accomplished, however, in development of preclinical fluorescent probes: both targeting and activatable probes have shown potential for translation to the clinic. The ideal probe is bright, features high specificity, offers strong signal-to-noise ratios, and is versatile (that is, it targets more than one specific cancer or disease). To attain these values, one needs to be aware of certain optical characteristics of tissue.
As live tissue is well perfused, light transparency is poor below 600 nm; the double bonds in the heme structure of hemoglobin significantly quench the light of these lower wavelengths. This phenomenon diminishes significantly in the NIR region (approximately 650–850 nm), where tissue becomes much more transparent to both excitation and emission wavelengths. Furthermore, in the lower wavelengths, tissue proteins autofluoresce, causing a significant amount of non-specific noise, disfavoring the signal-to-noise ratio. For these reasons, the window of opportunity is between 650 and 850 nm. Above that, water absorbs light and creates heat.
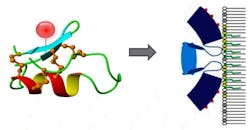
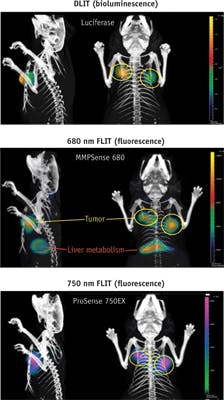
This window is large enough to facilitate multiplexing of 2–3 fluorescent probes in one subject. Other aspects to take into account are the reactive groups of the dye, its size, hydrophobicity, kinetics and clearance, and, of course, toxicity. Various targets such as the Annexin A2, as described above, have been validated. Other examples are probes that target and bind the hypoxia marker carbonic anhydrase IX, folate receptors, integrin αvβ3, Her2 (see Fig. 3) and the bombesin receptor.Activatable fluorescent imaging agents
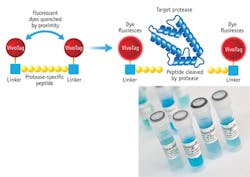
Binding moieties vary from small molecules to peptides to antibodies. A crafty approach to improve signal-to-noise ratios is the development of activatable fluorescent imaging agents. Non-fluorescent (optically silent) in their native (quenched) state, they generate high levels of fluorescence through enzyme-mediated release of their fluorochrome. These molecules are designed as follows: fluorochromes are attached to a backbone via specific linker peptides. When fluorochromes are held in close proximity, they quench each other. Upon excitation, specific proteases trigger proteolytic cleavage of these linker peptides. The fluorochromes are liberated and emit light, enabling visualization of tumor-specific protease activity. This strategy is highly sensitive, as a single enzyme cleaving multiple fluorescence agents results in unprecedented signal-to-background ratios to achieve several hundred-fold signal amplification (see Fig. 4).MMPSense is activated by key matrix metalloproteinases and MMP activity is involved in many disease-related processes, including cancer progression, invasion, and metastasis; rheumatoid arthritis; pulmonary diseases; and areas of cardiovascular disease. ProSense is activated by key disease associated proteases such as Cathepsin B, L, S, and plasmin, a marker for disease progression in animal tumor models as well as a marker for disease progression and therapeutic response in animal models of arthritis.
Other examples of activatable imaging agents are neutrophil elastase (this enzyme is a key protease involved in acute lung injury, acute respiratory distress syndrome, as well as many other inflammatory processes such as emphysema, cystic fibrosis, COPD, wound healing, rheumatoid arthritis, ischemia-reperfusion, and many others. ReninSense is cleaved by renin produced in the kidneys. The renin-angiotensin system (RAS) is the hormone system involved in regulating blood pressure and fluid balance in the body. This probe may be used to monitor abnormal RAS function, progression of disease, and the efficacy of therapeutic treatment in disorders such as hypertension and cardiovascular disease and some neurological diseases.
Beyond the development of probes for Tumor Paint practices to facilitate intrasurgical tumor resection, there is an imminent need for more advanced preclinical models with better clinical predictability. Kumar and colleagues1 have stated that "to protect pediatric subjects from life-threatening and/or debilitating adverse effects of drugs and to enhance the success rate in pediatric solid tumor trials, it is imperative that clinical trials be preceded by highly predictive preclinical investigations." For example, biomarker phenotyping of tumor cells can provide information regarding long-term response to therapy. An important feature of the most advanced mouse models is the inclusion of noninvasive imaging methodologies. Ideally, this imaging facilitates four activities—1) confirmation of tumor presence; 2) monitoring of tumor growth as a function of intervention, longitudinally without sacrificing; 3) interrogation of functional effects; and 4) assessment of biomarkers—all while significantly reducing the total number of animals required in a study.
In conclusion, mouse imaging technology has developed to a level of sophistication equal to the clinics. Optical molecular imaging is a popular methodology amongst the preclinical options and is just now finding applications into the clinical space as a valuable aid to surgical intervention. As the adaptation of clinical optical imaging continues to grow in parallel with the development of new optical contrast agents and imaging technologies, we foresee greater embracement of this modality by the medical field.
ACKNOWLEDGEMENTS
Tumor Paint is a trademark of Blaze Bioscience, Inc..
The author would also like to thank Blaze Bioscience, Inc. and the Washington State University Veterinary Teaching Hospital for their support with the publication of this article.
REFERENCE
1. S. Kumar, R. B. Mokhtari, H. Yeger, and S. Baruchel, Expert Opin. Drug Discov., 7, 11, 1093–1106 (Nov. 2012); doi:10.1517/17460441.2012.722077.
ALEXANDRA DE LILLE, DVM, Ph.D., is director of in vivo technical applications at PerkinElmer (Hopkinton, MA); e-mail: [email protected]; www.perkinelmer.com.