FLUORESCENCE/MEDICAL LASERS: Regulatory approval takes photonics-based systems to the clinic
Biophotonics-based systems earning recent regulatory approval include a welcome advance in colorectal cancer screening that gives patients a pass on the usual dietary restrictions and bowel prep. Also included is a laser-based adjunct to balloon angioplasty for peripheral artery disease, and a system for tattoo removal and treatment of pigmented lesions on all skin types.
A comfortable colo-cancer screen
Exact Sciences (NASDAQ:EXAS; Madison, WI) has received FDA approval for the first at-home, noninvasive test for colorectal cancer that analyzes both stool DNA and blood biomarkers using fluorescence. The test requires no medication, dietary restrictions, or bowel preparation, and demonstrated effectiveness in a prospective, 90-site, 10,000-patient pivotal study.1 The findings have "proven that this noninvasive test is highly sensitive in detecting both early-stage colorectal cancer and the most advanced precancerous polyps most likely to develop into cancer," said David Ahlquist, MD, a Mayo Clinic (Rochester, MN) gastroenterologist who co-invented the test. Mayo researchers developed the technology, and licensed it to Exact Sciences.
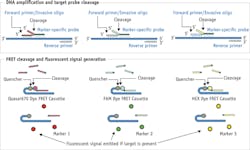
When a physician orders the Cologuard test, the kit is mailed directly to the patient's home. And once the patient collects a sample, he or she sends the kit to the Exact Sciences lab. There, Cologuard's Quantitative Allele-specific Real-time Target and Signal Amplification (QuARTS) technology looks for biomarkers that are shed from the colon as part of the digestive process, and blood released in the stool. Multiplexed QuARTS reactions are processed using a real-time cycler, with each biomarker (NDRG4, BMP3, KRAS, and ACTB) monitored separately through independent fluorescent detection channels. The sample is prepared and analyzed for fecal occult blood in a quantitative Enzyme-Linked Immunosorbent Assay (ELISA) that determines the concentration of hemoglobin in the sample.
Patients learn their results from their prescribing physician. The test is available in the U.S. for $599. The company plans to make Cologuard available in select countries in Europe pending CE Mark approval.
Laser-based adjunct to balloon angioplasty
Excimer laser maker Spectranetics (Colorado Springs, CO) has received FDA approval for its laser atherectomy products to treat in-stent restenosis (ISR; that is, return of blockage following stent placement) for patients with peripheral artery disease (PAD).
The development prompts a new standard of care in ISR treatment with improved clinical outcomes, and it follows clinical findings of the EXCImer Laser Randomized Controlled Study for Treatment of FemoropopliTEal (the arteries above and behind the knee) In-Stent Restenosis (EXCITE ISR). The study, reportedly the first multi-center, randomized prospective trial ever conducted for ISR treatment, demonstrated highly superior safety and efficacy of laser atherectomy with adjunctive percutaneous transluminal angioplasty (PTA, or "balloon angioplasty") compared with PTA alone. The trial shows a 94-percent procedural success rate using laser atherectomy with PTA vs. 83 percent with PTA alone.
In the study, the average lesion length was approximately 20 cm—compared to various stent IDE studies with average lesion lengths of 4 to 6 cm. Additionally, a high number of complex or advanced disease-state patients were enrolled in the trial, a fact that indicates success in treating all types of ISR lesions, including the most complex. Complete results from the EXCITE trial have been submitted to a peer-reviewed medical journal.
While stents deliver improved overall outcomes compared to PTA treatment, restenosis is common and stent re-obstruction or ISR remains therapeutically challenging. Once ISR develops, there is a 65-percent chance of recurrence after PTA, which is considered the standard of care. With over 115,000 ISR procedures performed annually in the U.S., Spectranetics says it is positioned to capitalize on potential market opportunities of $350 million domestically and up to $750 million worldwide.
Removing pigments from all skin types
Aesthetic laser system maker Syneron Medical (Irvine, CA) has received CE Mark approval for its dual-wavelength picosecond laser to remove tattoos and pigmented lesions on any skin type. Delivering 532 and 1064 nm wavelengths, the laser uses proprietary PicoWay technology to apply high peak power and ultrashort pulses for strong photomechanical impact. The company will begin a staged launch of the laser in the international market during the third quarter of 2014, and anticipates FDA clearance by the end of 2014.
REFERENCE
1. T. F. Imperiale et al., N. Engl. J. Med., 370, 1287–1297 (Apr. 3, 2014); doi:10.1056/NEJMoa1311194.
About the Author

Barbara Gefvert
Editor-in-Chief, BioOptics World (2008-2020)
Barbara G. Gefvert has been a science and technology editor and writer since 1987, and served as editor in chief on multiple publications, including Sensors magazine for nearly a decade.