NONINVASIVE BIOPSY/MICROSCOPY/SPECTROSCOPY: Optical innovations for cancer detection and treatment
DAN GAREAU
Quantitative endpoint metrics for diagnosis make bio-optical technologies attractive for pathology. And noninvasive measurement of biological tissues can safely and cost-effectively identify diseased or damaged tissue to guide treatment. Recent technology advances demonstrate the usefulness of reflectance-mode confocal microscopy (RCM) and optical fiber spectroscopy (OFS) for early-stage identification and treatment of skin cancer and ischemic injury, respectively.
The power of RCM
Confocal microscopy implements optical sectioning to produce noninvasive images that have similar resolution to that of histopathology—but the approach has the added benefits of being inexpensive, noninvasive, and fast. In addition, it is a digital technique, which enables convenient automated quantitative analysis.
In the context of biomedical imaging, the term confocal microscopy usually refers to fluorescence. But reflectance-mode confocal microscopy (RCM) can provide helpful information from specimens, and the technique requires minimal specimen preparation.1 For instance, RCM can aid in the diagnosis of the most fatal skin cancer, melanoma. Strong optical scattering from melanin granules provide endogenous contrast—which enables RCM to make visible the atypical melanocytes that indicate melanoma.2
Three key features of melanoma that RCM makes visible are: 1) the presence of pagetoid melanoma cells (PMs) in the epidermis, 2) the breakdown of the dermal/epidermal junction (DEJ) and 3) the absence of organized epidermal keratinocytes, which have respective sensitivity/specificity of 77.9%/69.7%, 89.7%/58.6% and 87.5%/52.1% using visual inspection.3 These traits are not easily identified by eye and so far, extensive confocal pathology training has been required to detect their presence. So RCM is a promising alternative. Thanks to a commercially available dermatological RCM system (the Vivascope 1500 by Lucid Inc.), and laboratory prototypes under development that aim to be 10X faster at half the price, RCM data is easy to acquire.
Automated RCM image analysis
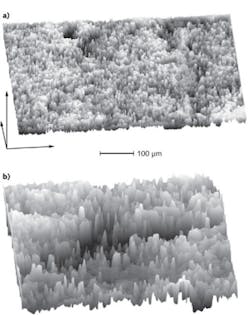
In an effort to make RCM data more useful to a wide range of dermatologists and pathologists, a research team at the Oregon Health & Science University (OHSU) developed an automated computer algorithm to operate on an RCM-produced 3-D digital image to yield a pathological diagnosis.4,5 The computer algorithm or “digital auto-pathologist” can detect PMs in superficial spreading melanomas (SSMs), and show that no PMs are present in benign birthmarks and moles (nevi). It can individually identify single melanoma cells in the epidermis, which overlies the DEJ, where melanoma is thought to arise. The DEJ, which otherwise has an undulating appearance (much like a landscape with rolling hills), appears broken (like a cityscape) when melanoma disrupts the normal pattern. The basal layer rests above—and hence traces the form of—the DEJ (see Fig. 1).
Melanoma can be detected by a large mean gradient, average slope or roughness “Ψ” between laterally adjacent points on the automatically located, pigmented basal surface. In the OHSU team’s first study, they found that for SSM, Ψ=11. 7±3.7µm^-1 versus a small Ψ=5.5±1.0µm^-1 for nevi (p = 0.0035). Visualizing the integrity (nevus) or broken, disjointed nature (melanoma) of this surface can provide invaluable diagnostic information to dermatologists.
Pattern recognition algorithms empower medical professionals with machine vision that is both tireless (and thus useful for sifting through large data sets) and able to detect subtle structural changes in tissue that can indicate the onset of disease and may be missed by visual inspection. It can also recognize the healthy epidermis that indicates melanoma is not present.5 The software able to process the 3-D images to render and analyze the diagnostic volume is under refinement with aims of commercialization and clinical translation. Challenges to bringing this technology to dermatology patients include expanding the studies to a wide range of melanomas and nevi, including difficult-to-detect melanomas such as amelanotic melanomas, which can appear as pinkish lesions and may not yield sufficient contrast under RCM, and moles such as Spitz nevi, which may show atypical features.
Confocal mosaics for staged excisions
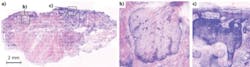
In addition to assisting in the identification of melanoma, confocal microscopy can facilitate routine removal of less deadly skin cancers, such as basal cell and squamous cell carcinoma. While at the Memorial Sloan Kettering Cancer Center (New York, NY), I developed a digital staining technique to support surgical procedures that combines reflectance- and fluorescence-mode confocal mosaics to replicate the appearance of histopathology.6 The difference is that the microscopy technique is noninvasive and painless, allowing for a better patient experience. In addition, it is 10X faster and more cost-efficient; in fact, it would save $150 million annually in the USA in Mohs surgery alone, and $8 billion if it were to entirely replace the current standard of care.7 Hematoxylin and eosin (H&E) histopathology, the current standard of care for detection, is expensive, requiring a laboratory, equipment and technicians; slow, requiring hours or even days to produce results; and at best only semi-quantitative.
Rapid confocal mosaic microscopy, which could perform the same task in as little as a few minutes, entails the sequential capture of adjacent image “tiles” that are stitched together to form images with both sub-cellular resolution and a wide field of view. Imaging in two modes (fluorescence for nuclei and reflectance for collagen) mimics the function of the stains hematoxylin, which highlights nuclei, and eosin, which highlights everything, particularly collagen. Hence, two imaging modes are used simultaneously to produce two gray-scale confocal mosaics, which can be effectively combined by adding color contrast. A natural choice for colors is the pink and purple of H&E already familiar to histopathologists. The result is an image that is similar to histopathology (see Fig. 2).Measuring oxygenation in tissue
Another technology under development at OHSU, OFS, uses spectral fingerprinting to identify ischemic injury to tissues. The OFS system monitors changes in the absorption spectrum between oxygen-bound and deoxygenated hemoglobin to gauge hypoxia.
Measuring the oxygenation of blood in mixed arteriovenous microvasculature to quantify tissue viability has the distinct advantage of not requiring pulsatile flow. Technologies in current use, such as pulsed oximetry and laser-Doppler flowmetry, require measurement of large pulsing arteries—whereas OFS works on whole tissue. Though many applications require assessment of oxygenation for guided treatment, surgeries often require modification of vasculature, running the risk of regional hypoxia (oxygen deprivation). Thus, a device and method for assessing tissue oxygenation in the microvasculature that lacks a strong pulsatile component is critically needed.
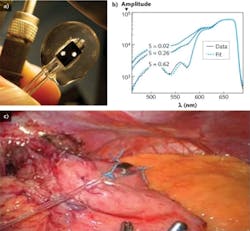
Diffuse steady-state OFS addresses this need.8 Recent studies have suggested that the oxygen saturation of tissue during surgery where vasculature is modified can be measured and used to predict surgical outcome.9 The team’s OFS device is a 12-foot fiber-optic probe that can be inserted into the patient’s abdomen to contact the tissue in danger of ischemia. A specialized probe tip guides light to and collects light from a 5 mm cubic sampling volume of tissue. The transmission spectrum in this sampling volume contains the spectral fingerprints of the chromophores present, namely oxyhemoglobin and deoxyhemoglobin. The reflectance spectrum is fit to a simulated spectrum, where the values of fraction blood volume and oxygen saturation are tuned for the least-squares error minimization (see Fig. 3).The ability to assess oxygenation and infer tissue viability may prove to be a critical diagnostic to indicate when a patient is in need of intervention before ischemic injury causes failure of surgically modified tissues.
Translation and future development
Quantum leaps that advance the state-of-the-art, such as rapid line-scanning as an alternative to conventional point-scanning RCMs, provide a rich base for clinical translation.10 But translation of technologies emerging from academia to the clinic also requires industry collaboration—so the team is seeking partnerships with companies manufacturing RCMs and pulsed oximetry units. Additionally, adoption of the OFS technology as a business model by Oregon State University’s MBA program has led to a trial company, “Phat Photonics,” that aims to put the device in the hands of a wide range of surgeons. Beyond strong industrial collaboration, innovation initiatives and so-called “gap funding” must increase for these technologies to bridge the gap to clinical translation. Market forces alone, for instance, will not support development of noninvasive optical biopsy if it prevents dermatologists from profiting by performing un-necessary biopsies.
One hardly needs a photomultiplier tube to see the light at the end of these research tunnels. The bright future of the confocal microscopy work includes developing automated optical therapy to treat cancer detected automatically. The spectroscopy diagnostic for ischemia may be augmented to survey wide areas, guiding the surgeon to manipulations that leave only viable tissue to heal.Spectroscopy may eventually be combined with the microscopy technology in a dermatology instrument able to detect the onset and progression of melanoma by measuring changes in molecular melanin populations. Some literature has suggested that there may be a shift from pheomelanin to eumelanin during the transition to vertical growth phase melanoma.11 While this theory is not well accepted, it exemplifies inroads being made into the molecular changes that may offer an optical fingerprint of the presence of disease. One successful example of such optical fingerprinting has been identification by Raman spectroscopy of the presence or absence of natural moisturizing factor in the skin as a sensitive/specific marker for the filaggrin gene mutation.12 Such sophisticated use of optics to measure the genetic traits of tissue is just one of many fronts on which bio-optics is advancing. Combination of the spectroscopy and microscopy technologies—in a dermatological instrument with spectral fingerprinting capabilities able to identify the onset and progression of early melanoma by changes in molecular melanin populations—will be pursued at Rockefeller University starting this year.
REFERENCES
1. S. Paddock, BioTechniques 32, 274 (2002).
2. M. Rajadhyaksha, J. Invest. Dermatol. 104, 946–952 (1995).
3. G. Pellacani et al., J. Invest. Dermatol. 127, 2759–2765 (2007).
4. D.S. Gareau et al., J. Biomed. Opt. 15 (6), 061713 (2010).
5. D.S. Gareau, J. Biomed. Opt. 16, 03052 (2011).
6. D.S. Gareau, J. Biomed. Opt. 14 (3) (2009).
7. D.S. Gareau et al., J. Biomed. Opt. 13 (5), 054001 (2008).
8. D.A. Benaron et al., J. Biomed. Opt. 044005-1 (2005).
9. D.S. Gareau et al., J. Biomed. Opt. 061712 (2010).
10. D.S. Gareau et al., Opt. Lett. 34 (20), 3235–3237 (2009).
11. R. Marchesini et al., J. Biomed. Opt. 14 (1), 014027 (2009).
12. Kezic et al., J. Invest. Dermatol. 128, 2117–2119 (2008).
Dan Gareau is a post-doctoral research fellow in the departments of dermatology and biomedical engineering at Oregon Health & Science University; [email protected]; www.ohsu.edu.