FLUORESCENCE MICROSCOPY/NEUROSCIENCE/DRUG DEVELOPMENT: Glowing molecules, operationally enhanced by time, promising for Alzheimer's work
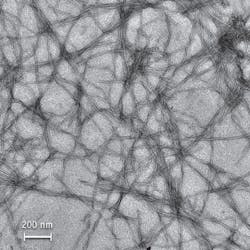
Metallic molecules that naturally attach to beta amyloid proteins called fibrils are a subject of interest to Rice University researchers for two reasons: Fibrils form plaques in the brains of people with Alzheimer’s disease—and when the metallic molecules connect to them, they glow 50 times brighter. What’s more, the particular way the molecules glow could prove useful for drug development. Because of the way they work, the complexes could facilitate work with fluorescence lifetime imaging microscopy (FLIM).
The overall effect may provide an alternative current method of studying the fibrils, which seem to form when misfolded proteins begin to aggregate. The standard approach to amyloid fibril research involves use of Thioflavin T (ThT) dyes. But ThT fluoresces only when excited at 440 nm, and it emits light at 480 nm—providing only a 40 nm window, or Stokes shift. Because some drugs are also fluorescent, they can obscure the fluorescence of ThT, and thus make assays unreliable.”In the case of our metal complexes, the Stokes is 180 nm,” said Angel Martí, an assistant professor of chemistry and bioengineering. “We excite at 440 and detect in almost the near-infrared range, at 620 nm. That’s an advantage when we want to screen drugs to retard the growth of amyloid fibrils.”
Graduate student Nathan Cook also exploited the metallic molecule’s long-lived fluorescence by “time gating” spectroscopic assays. “We specifically took the values only from 300 to 700 ns after excitation,” he said. “At that point, all of the fluorescent media have pretty much disappeared, except for ours. The exciting part of this experiment is that traditional probes primarily measure fluorescence in two dimensions: intensity and wavelength. We have demonstrated that we can add a third dimension—time—to enhance the resolution of a fluorescent assay. This aspect makes the complexes appropriate for use with FLIM, which discriminates among microenvironments based on the length of a particle’s fluorescence rather than its wavelength.”
1. N.P. Cook et al., J. Am. Chem. Soc. 133, 29, 11121–11123 (2011).
More BioOptics World Current Issue Articles
More BioOptics World Archives Issue Articles