STORM made easy for high-throughput research
Single-molecule localization microscopy (SMLM) methods (PALM,1,2 PAINT,3 and STORM4,5) have become important tools to access biological information at the nanoscale. These techniques can resolve structures with a quasi-isotropic 3D localization precision of ~10 nm.6 However, SMLM is reliant on fluorophore photoswitching characteristics,7 which depend on the wavelength and significant chromatic aberration at the nanoscale. These challenges are hampering straightforward, multicolor imaging with SMLM. So far, the multicolor information has been accessed by sequential acquisitions at different excitation wavelengths, which on the other hand induce uncontrollable drifts between the different color recordings.
But now, researchers have demonstrated a novel solution where only a single laser is used to excite multiple fluorophores in the same spectral range (far-red) coupled to a spectral demixing strategy that overcomes these limits and permits 3D multicolor nanoscopy with increased simplicity, speed, and reliability of output data.
SAFe RedSTORM strategy
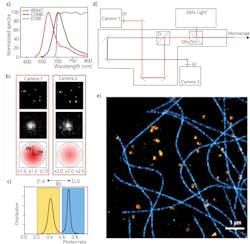
Different methods have been proposed by the SMLM community, based on a spectral demixing algorithm.8-13 By taking these approaches one step forward, it has recently been demonstrated that an easy and robust ratiometric technique can be used to spectrally separate the fluorescence emission contributions of red-emitting dyes; this allows for multicolor imaging. With this new technique, the fluorescence emission light is split with a long-pass dichroic (for example, at 700 nm) into two separate pathways before reaching the two cameras (see Figs. 1a and 1d). The photon counting is measured for each detection on both cameras and the calculated photon ratio—R—is related to the spectral separation; R thus represents the ƛ identity card (see Fig. 1c). Two parameters have a main role at this point of the process: the range chosen for ƛ-assignment and the percentage of rejected molecules. The goal is to minimize crosstalk to best separate different structures, while still rejecting as little as possible of the emission detections for good-quality reconstruction.
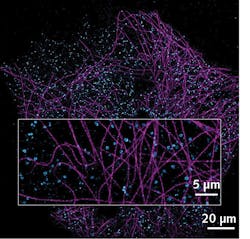
An example of achieved multicolor nanoscale imaging is shown in Figure 1e—this is a reconstruction of a Cos7 cell labeled for tubulin AF647 and Clathrin CF680, and excited with a single high-power 640 nm laser. This new spectral de-mixing strategy is implemented in Abbelight’s dedicated system, SAFe RedSTORM, as well as in the highest-range Abbelight SAFe360. Both modules can be adapted to any inverted microscope, from simple widefield microscopes to more complex confocal or spinning-disk configurations, to turn microscopy into nanoscopy.The main advantage with this spectral de-mixing approach is that only one laser is required for two-color excitation, as the fluorescent dyes used are all excited in the same spectral region. Furthermore, chromatic aberrations are reduced and there is no need for drift correction between different excitation colors—the wavelength information is recorded in one acquisition. The easiness and robustness of the process is such that straightforward multicolor imaging at the nanoscale is gained both in 2D and 3D, ensuring a maximal crosstalk of 10% in the worst-case scenario for three dyes in 3D (with typical values kept below 1% crosstalk for two dyes in 2D).
SAFe RedSTORM imaging for neuroscience
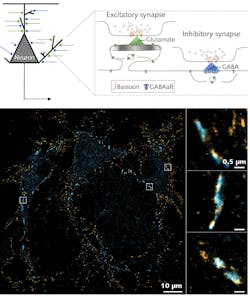
Super-resolution microscopy has emerged as a powerful tool to understand fundamental neuronal functions such as the transmission of information between neurons at synapses. The key proteins involved are precisely organized and this nanoscale organization fine-tunes synaptic transmission efficiency. However, measuring this precise alignment between the presynaptic release machinery and the postsynaptic receptors can be difficult due to chromatic aberration. Spectral de-mixing multicolor SMLM can therefore be a powerful tool to study such precise co-organization.
To investigate these capabilities of multicolor SMLM in neuroscience, Dr. Benjamin Compans and his colleagues at the Burrone Lab at King’s College London studied two proteins involved in synaptic transmission. Bassoon and GABAa receptors (GABAaRs) were labeled with AF647 and CF680, respectively, and imaged in an Abbelight SAFe RedSTORM system. Bassoon is a presynaptic scaffolding protein that assembles the active-zone site where neurotransmitters are released. It can be found at both excitatory and inhibitory synapses. GABAaRs are the main effector of the inhibitory synaptic transmission—they are stabilized at the postsynaptic membrane in front of the GABA release site and are activated upon GABA release. Combined large FOV and super-resolution multicolor imaging of these proteins are shown in Figure 4.Lasers for SMLM
In SMLM, high irradiance (several kilowatts per square centimeter) is crucial to reach a single-molecule regime and ensure quantitative imaging at the nanoscale. It becomes even more critical while imaging three different fluorophores simultaneously at the density needed for SMLM over large FOVs. Furthermore, as higher irradiance increases the blinking rate, increased irradiance allows for faster imaging at the nanoscale.
The need for high irradiance to excite multiple fluorophores with nanoscale resolution over large FOVs and at high speeds translates into the need for high-power laser sources. In this regard, the availability of new, compact, powerful laser sources in the red spectral range is facilitating the development of simplified multicolor SMLM imaging technology with high-throughput capability.
The Cobolt Rogue continuous-wave (CW) laser, at 640 nm and with 1 W, is suitable for simultaneous excitation of red dyes, such as AF647 and CF680. The diode-pumped laser emits a diffraction-limited, single-transverse-mode TEM00 beam, which allows efficient coupling into single-mode polarization-maintaining fibers for efficient delivery into the microscope.
The laser operates in multi-longitudinal mode in combination with low intensity noise; this is fairly unusual for diode-pumped laser technology in the visible spectral range. Typically, diode-pumped CW lasers in the visible are based on intracavity frequency conversion and in such configurations. It is important to operate the laser on one single-longitudinal cavity mode to avoid the intensity noise arising from chaotic fluctuations in nonlinear losses between competing modes in a multi-longitudinal-mode cavity—so-called “green noise.”
Single-longitudinal-mode (SLM, or single-frequency) operation is usually achieved by intracavity spectral filtering mechanisms, which to some extent reduces the output power. As the Cobolt Rogue is operating directly at the fundamental wavelength and without frequency conversion, it is possible to operate the laser in multi-longitudinal mode and still with low-intensity noise. This means spectral filtering is not necessary, and thus allows power scaling of the output.
The Cobolt Rogue laser is based on Pr3+-doped laser crystals, which is a relatively new class of diode-pumped laser sources on the market. The realization and industrialization of this laser technology has been made possible thanks to developments in pump diode technology in the visible range. An advantage with these lasers is that they emit directly in the visible range, which means the output power can more easily be scaled in small formats by operating the laser in multi-longitudinal mode while still emitting a perfect single-transverse-mode beam. Another advantage with the multi-longitudinal mode operation in imaging applications is that the broader optical spectrum reduces the coherence length, which in turn reduces the impact of speckle effects on image quality or interference from surfaces in the beam path. This is of particular importance in imaging applications where larger areas are illuminated.
By using a laser technology that emits directly in the visible, in a multi-longitudinal mode configuration and combined with an assembly technology based on high precision mounting of miniaturized optics onto thermally controlled, hermetically sealed platforms, output powers of 1 W can be achieved in a perfect TEM00 beam with a very high level of output stability, even in varying ambient conditions, from a compact laser housing size of 115 × 55 mm.
Outlook
The combination of a compact high-power red laser output delivered via a single-mode polarization-maintaining fiber with Abbelight’s SAFe RedSTORM technology, which is focused on a ratiometric approach to spectrally separate the fluorescence emission contributions of red-emitting dyes, has enabled the realization of a multicolor 3D nanoscopy capable of delivering images at the nanoscale over large FOVs (up to 200 × 200 µm2) and with nanometer localization precision (15 nm). It’s easy to integrate and use, and therefore broadly accessible.
REFERENCES
1. E. Betzig et al., Science, 313, 1642–1645 (2006); http://doi.org/10.1126/science.1127344.
2. S. T. Hess et al., Biophys. J., 91, 4258–4272 (2006); http://doi.org/10.1529/biophysj.106.091116.
3. A. Sharonov and R. M. Hochstrasser, Proc. Nat. Acad. Sci., 103, 18911–18916 (2006); http://doi.org/10.1073/pnas.0609643104.
4. M. J. Rust et al., Nat. Methods, 3: 793–795 (2006); https://doi.org/10.1038/nmeth929.
5. M. Heilemann et al., Angew. Chem. Int., 47, 6172–6176 (2008).
6. L. Möckl and W. E. Moerner, J. Am. Chem. Soc. (2020); https://doi.org/10.1021/jacs.0c08178.
7. G. T. Dempsey et al., J. Am. Chem. Soc., 131: 18192–18193 (2009); https://doi.org/10.1021/ja904588g.
8. M. Bossi et al., Nano Lett., 8, 2463–2468 (2008); https://doi.org/10.1021/nl801471d.
9. I. Testa et al., Biophys. J., 99, 2686–2694 (2010); http://doi.org/10.1016/j.bpj.2010.08.012.
10. D. Baddeley et al., PLoS One, 6, 5, e20645 (2011); https://doi.org/10.1371/journal.pone.0020645.
11. A. Lampe et al., Biol. Cell, 104, 229–237 (2012); https://doi.org/10.1111/boc.201100011.
12. C. M. Winterflood et al., Biophys. J., 109, 1, 3–6 (2015); https://doi.org/10.1016/j.bpj.2015.05.026.
13. R. Diekmann et al., Nat. Methods, 17, 9, 909–912 (2020); https://doi.org/10.1038/s41592-020-0918-5.
14. A. Mau et al., Nat. Commun., 12, 3077 (2021); https://doi.org/10.1038/s41467-021-23405-4.
About the Author
Valentina Caorsi
Head of the R&D Department, Abbelight SAS
Dr. Valentina Caorsi is Head of the R&D Department at Abbelight SAS (Cachan, France).
Håkan Karlsson
CEO and Cofounder, Cobolt (HÜBNER Photonics)
Håkan Karlsson is CEO and cofounder of Cobolt, a part of HÜBNER Photonics (Solna, Sweden).