Brain-machine interfaces (BMIs), devices that connect neural signals to external devices, control prosthetics and also do the inverse: deliver signals from an external device for the brain to interpret. An ideal BMI would enable two-way communication to mimic the closed-loop performance of brains in living organisms, where sensory feedback and motor control occur simultaneously.
Optical technologies are key to more capable BMIs because they are fast, relatively noninvasive, and noninterfering with each other or with electrical signals. Although BMIs were originally conceived as mechanisms to exploit knowledge of neurology for practical ends, they are now offering insights into neural plasticity, neuropathology, and the fundamental relationship between physiology and cognition.1 BMI technology is rapidly advancing on multiple fronts to allow more rapid data transmission, operate on finer scales, and create entirely new capabilities.
Ramping up the speed
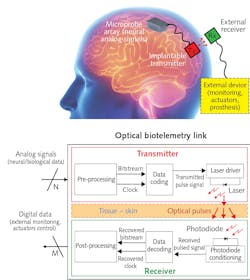
Future implantable devices may be capable of detecting a dense array of single-neuron signals, but how should the data be transmitted? Professor Andrea De Marcellis and his colleagues at the University of L’Aquila in Italy and Imperial College London sought implementing optical communication in an implantable biotelemetry solution inspired by ultra-wideband pulsed data coding.2 De Marcellis says his approach offers “a high data transmission rate, low power consumption, and lack of electromagnetic interference.”
The researchers designed a system around an 850 nm VCSEL transmitter and a 250-µm-diameter photodiode with a 47 ps response time, coupled to a high-gain amplifier. To minimize transmission errors without increasing laser power—and without risking tissue heat damage—they implemented synchronous on-off key modulation, combining clock and data signals to quickly and automatically maintain data integrity.
To validate the scheme and find the optimum operational parameters, they simulated neural signals for transmission through a VCSEL nestled against the interior side of a 3.5-mm-thick section of porcine skin with a photodetector on the opposite side (see Fig. 1). With 900 ps pulses at 300 MHz and an average optical power of less than 2 mW, the system demonstrated a bit-error rate lower than 10-10 with a total electrical power usage of less than 11 mW.
De Marcellis’ team has implemented this protocol in an integrated ASIC system-on-chip (SoC) and is currently studying possible optoelectronic solutions to improve misalignment tolerance and reduce power requirements.3 “Our optical biotelemetry is particularly suitable for highly scalable implantable devices, such as brain machine interfaces,” he said.
From one mouse to another
Optical technology improves data transmission speed and quality, but that doesn’t help with acquiring neural signals, nor does it help apply those signals in some useful fashion. Luhui Liu and Ruiyu Wang in Minmin Luo's Lab at the National Institute of Biological Sciences in Beijing, China, are addressing that challenge in dramatic fashion. They have acquired signals from the locomotor region of one mouse and stimulated another mouse’s brain, causing the second mouse to walk synchronously with the first.4
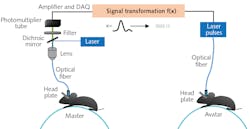
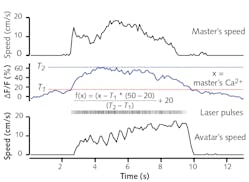
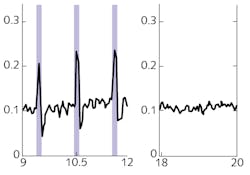
Luo’s team optogenetically modified neurons in the locomotor control section of a “master” mouse to express the genetically encoded Ca2+ indicator GCaMP6. Particularly, they targeted specific neurons in the nucleus incertus (NI) region—neurons that express the neural signaling molecule neuromedin B (NMB). In earlier studies, they had discovered those neurons were implicated in directing locomotion, among other things. They then implanted an optical fiber to illuminate and interrogate that brain region. When excited by 488 nm light, GCaMP6 fluoresces at high levels when in the presence of Ca2+ ions; fluorescence becomes an indication of neural activation. They head-fixed the mouse to the apparatus and allowed it to move at-will on a treadmill. Brighter fluorescence, indicating higher neural activation, correlated with faster motion.
The researchers also modified NI NMB neurons in another mouse, an “avatar” mouse. That modification was inverse—the mouse was modified so neuron activity could be optically triggered. Specifically, these neurons were transfected to express channelrhodopsin-2 (ChR2), making them sensitive to light. They implanted a fiber in the brain of the avatar mouse so it could illuminate the NI. When illuminated with 5 ms pulses of 488 nm light at frequencies of 5, 10, 20, and 50 Hz, the avatar mouse—head-fixed in the same fashion as the master mouse—walked on a treadmill in response to the optical signal (see Fig. 2).According to Luhui Liu, a postdoc on the team, “we have demonstrated that an optical brain-to-brain interface (BtBI) can transfer locomotion information at a transfer rate at least two orders of magnitude higher than the estimated information rates reported for EEG-based BtBIs.” With multiple fibers interrogating the master mouse, and the addition of co-expressed optical probes, it may be possible to localize finer locomotor control signals, such as those operating turning or backwards motion. This work opens the remarkable possibility of future developments of “BtBIs that allow one to fully control the locomotion of other individuals,” Liu says, “within or even across species.”
Directly from the brain
The previously described developments imply or require that some external mechanism to make neural signals is accessible outside the brain. But what if the brain itself could deliver its internal signals to a place accessible to the outside world? Moves in that direction have been taken by Professor D. Kacy Cullen of the University of Pennsylvania. He and his colleagues, as part of a multi-institution team, have developed a “living electrode” from optogenetically modified neurons—an electrode capable of translating signals from within the brain to the surface of the skin.5The researchers then implanted µTENNs in the primary visual cortex of rats, with the other end extending to the skull surface, where it was protected by a sealed window. Using multiphoton microscopy, they could visualize neural activation through the calcium-linked RCaMP1b fluorescence. The µTENNs remained viable for at least a month. Even more dramatically, immunohistochemistry of the rat brains showed that the µTENN neurons extended a few millimeters from the implantation site as well as clear evidence of synaptic formation with the rat brain.
Although this aspect of the work is in its early stages, some implications are already clear. The “living electrode” formed by a µTENN can provide direct surface access to neural signals from within the brain, laying the groundwork for future neural interfaces. The researchers conclude that “multiple distinct µTENNs could also be grown in proximity to form connected assemblies … to model, manipulate, and characterize systems-level network dynamics across neuronal circuits.”
REFERENCES
1. K. A. Moxon et al., Neuron, 86, 1, 55–67 (2015); doi:10.1016/j.neuron.2015.03.036.
2. A. De Marcellis et al., IEEE Trans. Biomed. Circuits Syst., 14, 3, 441–451 (2020); doi:10.1109/tbcas.2020.2972733.
3. A. De Marcellis et al., Proc. IEEE, 1–5 (2020); doi:10.1109/iscas45731.2020.9180609.
4. L. Lu et al., Sci. China Life Sci., 63 (2020); doi:10.1007/s11427-020-1675-x.
5. D. O. Adewole et al., Sci. Adv., 7, 4, eaay5347 (2021); doi:10.1126/sciadv.aay5347.