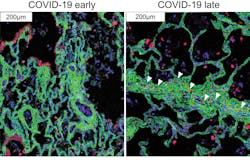
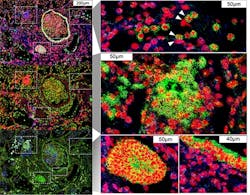
Imaging cytometry method details lung pathology in COVID-19
Researchers at Weill Cornell Medicine and NewYork-Presbyterian (both in New York, NY) used a imaging cytometry method to map, at single-cell resolution, the cellular landscape of diseased lung tissue in severe COVID-19 and other infectious lung diseases.
In the study, the research team imaged autopsied lung tissue in a way that simultaneously highlighted dozens of molecular markers on cells. Analyzing these data using novel analytical tools revealed new insights into the causes of damage in these lung illnesses and a rich data resource for further research.
“COVID-19 is a complex disease, and we still don’t understand exactly what it does to a lot of organs, but with this study we were able to develop a much clearer understanding of its effects on the lungs,” says co-senior author Dr. Olivier Elemento, professor of physiology and biophysics, director of the Caryl and Israel Englander Institute for Precision Medicine, associate director of the HRH Prince Alwaleed Bin Talal Bin Abdulaziz Alsaud Institute for Computational Biomedicine at Weill Cornell Medicine and co-Director of the WorldQuant Initiative for Quantitative Prediction, which funded the technology for single cell analysis of tissue. “I think the technological approach we used here is going to become standard for studying such diseases.”
Traditional tissue analysis, often using chemical stains or tagged antibodies that label different molecules on cells, can reveal important features of autopsied tissues. However, this approach is limited in the number of features it can mark simultaneously. It also usually doesn’t allow detailed analyses of individual cells in tissues while retaining information about where the cells were in the tissue.
However, the method that the research team used—imaging mass cytometry—largely overcomes those limitations. It uses a collection of metal-tagged antibodies that can simultaneously label up to several dozen molecular markers on cells within tissues. A special laser scans the labeled tissue sections, vaporizing the metal tags, and the metals’ distinct signatures are detected and correlated with the laser position. The technique essentially maps precisely where cells are in the sample, as well as each cell’s surface receptors and other important identifying markers. Altogether, over 650,000 cells were analyzed.
The researchers applied the method to 19 lung tissue samples autopsied from patients who had died of severe COVID-19, acute bacterial pneumonia, or bacterial or influenza-related acute respiratory distress syndrome, plus four lung tissue samples autopsied from people who had had no lung disease.
The findings in samples from COVID-19 cases were broadly consistent with what is known about the disease, but clarified this knowledge in much finer detail. They showed, for example, that cells called alveolar epithelial cells, which mediate the lungs’ gas-exchange function, are the main targets of infection by SARS-CoV-2, the coronavirus that causes COVID-19. The analysis suggested that these infected cells are not solely singled out for attack by lung-infiltrating immune cells, which may help explain why inflammation often keeps worsening in severe COVID-19 and ends up causing such extensive and relatively indiscriminate damage.
The researchers found that age and sex, two major factors in mortality risk for COVID-19, made no apparent difference at the histologic level, once COVID-19 had progressed to the severe stage.
The results also showed that white blood cells called macrophages are much more abundant in the lungs of severe COVID-19 patients compared to other lung diseases, whereas white blood cells called neutrophils are most prevalent in bacterial pneumonia—a distinction that may be relevant to the development of future treatments for these infectious diseases.Overall, the study provides a fine-grained picture of the disease process in COVID-19 and how it differs from other infectious lung diseases. It has prompted new research questions that are now being investigated, the investigators say, and includes a wealth of observations that wouldn’t have been possible with standard pathology techniques.
“The application of technology like what we’ve demonstrated here is going to provide a huge boost to the utility of autopsy-based studies of disease,” said co-senior author Dr. Alain Borczuk, professor of pathology and laboratory medicine at Weill Cornell Medicine and a pathologist at NewYork-Presbyterian/Weill Cornell Medical Center.
The researchers emphasized that the technique not only will be applicable to a broad set of other diseases for which tissue can be obtained, but also should give doctors and scientists a practical method for delineating important differences within disease categories.
“Traditionally for lung, liver, and other organ diseases, we have these broad diagnoses that in fact cover multiple distinct diseases—now we have a tool that that will enable us routinely to distinguish among these different diseases, and hopefully make use of those distinctions in treating patients more effectively,” says co-senior author Dr. Robert Schwartz, an associate professor of medicine in the Division of Gastroenterology and Hepatology at Weill Cornell Medicine, and a pathologist at NewYork-Presbyterian/Weill Cornell Medical Center.
Full details of the work appear in the journal Nature.