LIBS finds a niche for portable applications
Ken Bell
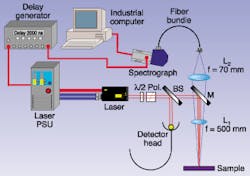
Although not as precise as some spectroscopic methods, laser-induced breakdown spectroscopy allows straightforward spectral analysis in the field.Laser-induced breakdown spectroscopy (LIBS) is a powerful tool that allows detection and measurement of elemental species in a variety of matrices. The principle is simple. A laser beam, commonly (though not always) from a pulsed Q-switched Nd:YAG laser, is focused onto the sample of interest and the high instantaneous peak irradiance generates a plasma or spark at the surface (see Fig. 1). Since the temperature in the plasma is typically around 10,000 K, the species present are turned into electronically excited atoms and ions. As the excited species decay, they emit radiation, typically in the ultraviolet to near-infrared range, in a characteristic spectrum. Light from the spark is gathered and analyzed using a spectrophotometer (see Fig. 2). The signal light is weak in comparison to the plasma-inducing laser pulse, so a highly sensitive, gated detector is used with a filter to eliminate the laser light. The detector is typically either a charge-coupled-device array, a camera, or, in rare cases, a photomultiplier. Intensified detectors are a common choice. Cameras and arrays can give information on a range of wavelengths at once, relying on the known angle-wavelength relationship of the spectrometer.
Different spectrometers provide different results. Conventional Czerny-Turner spectrometers range over a few nanometers in wavelength, while an échelle device provides information on a much wider wavelength range. A photomultiplier is unable to resolve wavelengths and so is typically used to look at one particular wavelength, corresponding to a specific species. Varying detector gate times and delays in a LIBS system can yield additional information. At the end of the laser pulse, the hot plasma consists of atoms and highly charged ions, which decay and cool over a period of microseconds back to neutral atoms and then a solid and/or gaseous condensate. By selecting an appropriate time window during this period, different information may be obtained, from the plasma continuum early on, to the discrete elemental spectra later.
Gaining quantitative information
Qualitative information about the elements present in a sample is easy to find by studying the spectral characteristics of the system output. Quantitative information is more difficult to obtain; typical precision with LIBS is in the range of 5% to 20%.
The amplitude of a signature spectral feature is dependent on several factors. To build up a spectrum with a good signal-to-noise ratio, as well as set the appropriate gate time, the sample is typically exposed to numerous laser pulses. As time goes by, these tend to form a crater in solid samples, leading to a larger plasma plume and a stronger signal. Ratio techniques must then be used to allow for the changing amplitude of the signal, by comparing the ratio of the signals from various species present. Moreover, this ratio can be dependent upon laser energy; studies show that use of lower laser energies gives an energy-dependent ratio, which stabilizes at higher energy levels. When the incident energy density is low, the composition of the plasma depends more on the relative volatility of different species than the actual concentrations. At high energy densities, nearly everything in the vicinity of the point of impact is vaporized. Thus, the signal strength for a particular species depends on concentration as well as on the matrix containing it. To derive quantitative information, the signal must be compared to another sample of a similar matrix in which the concentration of the species to be measured is already known or with a sample in which another species exists in constant concentration.
A further cause of uncertainty is the atmosphere surrounding the sample. While it is preferable to use controlled atmospheric conditions to eliminate these effects, the demand for user-friendly, field-portable instruments means that this is not always practical. Fortunately, in many cases, such as in air, the variations are not so great.
An overview of LIBS
Compared to other elemental analytical techniques such as laser-ablation inductively coupled plasma mass spectroscopy (LA-ICPMS), LIBS requires little or no sample preparation, and optical spectrometers are simpler and smaller than mass spectrometers. Machines for LA-ICPMS normally require laboratory conditions and careful sample preparation. However, LA-ICPMS can detect species in parts-per-billion concentrations, compared to typical concentrations in parts per million for LIBS, making the former a much more sensitive technique. Mass spectroscopy relies on charged species separating according to their charge-to-mass ratio in a magnetic field before hitting a detector. Therefore, the sensitivity of the technique is broadly similar for all species.
The LIBS technique relies on the electronic-decay emission characteristics of different species, leading to great variations in sensitivity. For example, silver, copper, and chromium can be detected in concentrations of a few parts per million; sulfur and iron are detectable in concentrations of hundreds of parts per million. These figures can vary widely, depending on the surrounding matrix. Moreover, the presence of other elements with overlapping spectral characteristics can affect results. In x-ray emission spectroscopy, a sample irradiated by an electron beam emits a characteristic x-ray spectrum to measure metal concentrations very precisely but, unlike LIBS, requires more highly specialized equipment and is suitable only for metallic species. The spatial resolution of LIBS is in the range of a few tens of microns, similar to that of the other techniques mentioned here.
Applications of LIBS
As a result of efforts in the scientific community, the last few years have seen greater understanding of and refinements to the LIBS technique. Several companies produce commercially available LIBS instruments. The availability of compact, rugged, pulsed Nd:YAG laser sources has enabled LIBS to be carried from the laboratory into the real worldincluding such places as chemical processing plants, mines, metal refineries, and even nuclear reactors (see Fig. 3).
Many chemical processing and manufacturing applications involve the production of toxic effluents. The LIBS technique monitors the concentration of toxic elements in the effluents to ensure they remain within safe and legally permissible concentrations. Because sample preparation is not necessary and results can be obtained quickly or even in real time, online monitoring is possible, reducing the need to store effluent while laboratory results are obtained and lowering the risk of environmental damage.
In metal refineries, certain elements, even in modest concentrations, can seriously affect the properties of metals, both detrimentally and positively. Samples removed from blast furnaces and smelters are routinely analyzed in a laboratory using ICPMS and x-ray emission spectroscopy. By the time analysis is complete, an entire batch of metal may have solidified. Using LIBS for a real-time analysis of the molten material allows any required remedial action to be taken promptly.
In the mining industry, LIBS can determine concentrations of valuable minerals in the vicinity. This enables a quick decision regarding whether to take away ore for smelting or to discard it. For minerals existing in small and variable concentrations, the economic and environmental benefits of not having to mine, transport, and attempt to refine poor-grade ore are significant.
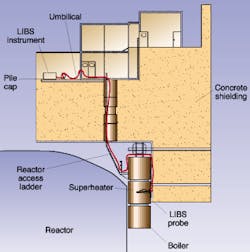
The nuclear industry relies on materials analysis to monitor its reactors and predict failures. Removing samples from highly radioactive environments is by no means desirable for safety and operational reasons. An onsite LIBS instrument equipped with umbilical fiberoptics can remotely monitor changes in metallurgical composition that predict failure in reactor components (see Fig. 4).
Other LIBS applications include the study of ancient works of art. With its high spatial resolution, LIBS can probe layers of paint providing information on their composition. In the pharmaceutical industry, LIBS also can probe tablets in order to determine the concentration and distribution of active drug compounds quickly and fairly accurately.
The future of LIBS
While LIBS is gaining acceptance as a real-world analytical technique, it is still the subject of development in universities, research institutions, and commercial laboratories around the world. Most of the current techniques are based on Q-switched Nd:YAG lasers operating at their fundamental wavelength of 1.06 µm, producing tens of millijoules in pulses of about 10 ns. Such lasers are commercially available and are rugged and reliable enough to be used anywhere. The LA-ICPMS technique uses the ultraviolet harmonics of Nd:YAG lasers as well as the fundamental wavelength. Use of other, easily accessible Nd:YAG wavelengths is likely to become more commonplace in the race to refine the process. Ultrafast lasers with femtosecond pulses hold promise for time-resolved techniques because they interact very differently with materials. Observing the spectral output shortly after the pulse yields subtle information about sample composition.
Laser-induced breakdown spectroscopy provides researchers and technicians with a versatile analytical tool that is easy to use. The technique produces good qualitative information quickly, with moderate quantitative precision. Other techniques offer higher sensitivity and precision but require specialized, nonportable equipment and a higher degree of sample preparation. In the future, LIBS developments may bring about an increase in precision without sacrificing convenience or speed.
KEN BELL is director of sales and marketing at Big Sky Laser Technologies Inc., P.O. Box 8100, Bozeman, MT 59715-2001. E-mail: [email protected].