Cytometry/Spectroscopy: Optics and photonics advance spectral flow cytometry
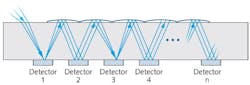
![FIGURE 1. Flow cytometry passes a stream of labeled cells in front of laser beams and an objective lens collects light emitted by the cells. In conventional systems, the laser wavelengths are matched to excite the cells’ labels, and the light collected by the objective passes through dichroic mirrors and filters to PMTs. In spectral systems, light from each of the lasers illuminates all of the labels, and the earliest of these systems featured dispersive optics that distributed fluorescence emission onto linear detector arrays. (Adapted from http://nolanlab.com/spectral-fc.html [4]) FIGURE 1. Flow cytometry passes a stream of labeled cells in front of laser beams and an objective lens collects light emitted by the cells. In conventional systems, the laser wavelengths are matched to excite the cells’ labels, and the light collected by the objective passes through dichroic mirrors and filters to PMTs. In spectral systems, light from each of the lasers illuminates all of the labels, and the earliest of these systems featured dispersive optics that distributed fluorescence emission onto linear detector arrays. (Adapted from http://nolanlab.com/spectral-fc.html [4])](https://img.laserfocusworld.com/files/base/ebm/lfw/image/2019/02/content_dam_lfw_print_articles_2019_02_1902lfw_bg_f1.png?auto=format%2Ccompress&w=250&width=250)
Cytometry (literally, cell measurement) encompasses the assessment of such characteristics as cell size, shape, and structure; quantities of cell populations; functional cellular states; and constituents such as specific proteins and their locations, and DNA. It is a mainstay for both cell biology and clinical applications, including common blood tests and disease diagnosis.
A fluorescence technique that uses a laser light to measure cell characteristics in a fluid stream, flow cytometry has advanced over the last few decades through innovation in hardware and reagents by numerous engineers and researchers. Their collective work has culminated in the current ability to perform multiparameter analysis of individual cells at throughput rates of tens of thousands of cells per second.
But while the development of fluorophores has resulted in a tantalizing palette of options, managing this abundance is a challenge that requires careful choice of colors and filters. Not only are specialized dyes often required with conventional flow cytometry, but adding colors makes it easy for dim stains to become masked by spectral spillover, and dyes with close or overlapping spectral profiles are difficult to differentiate, which limits resolution.
Conventional flow cytometry requires multiple lasers, uses dichroic mirrors to produce independent signal detection paths, and follows a paradigm of one filter and one detector (usually a photomultiplier tube [PMT]) per dye. So, adding colors means increasing hardware and panel design complexity.
What’s more, flow cytometry is challenged to identify cells from different tissues and organisms, requiring large, multiparametric compensation matrices for data analysis.1
Just add spectroscopy
Aided by recent advances in detectors, optics, and computation that enable submillisecond, full spectral measurements,2 spectral (or multi- or hyperspectral) flow cytometry has emerged in recent years to address these limitations. Spectral flow cytometers, which incorporate ultrafast optical spectroscopy,3 have simpler optical paths and fewer components than conventional flow cytometers, and they provide higher quality results with fewer lasers.
In both forms of flow cytometry, fluorescently labeled cells in suspension pass one by one in front of laser beams with wavelengths matched to excite their labels. In conventional flow cytometry, each of the lasers can excite 3–5 colors—the optical system includes an objective lens that collects light emitted by the cells and directs it through dichroic mirrors and filters to detectors. In spectral flow cytometry, each of the system’s lasers excites all the labels used. Some of the first spectral approaches use dispersive optics such as prisms and gratings to distribute fluorescence signal (from organic dyes, nanoparticles, and fluorescent proteins within each cell) onto linear detector arrays (see Fig. 1).3 A more recent innovation, aided by advances in semiconductor detectors, telecom optics, and computation, has enabled full-spectral measurement with >24 colors for high-quality data output using just three lasers (see Fig. 2). This latest approach to has led to the acceptance of spectral cytometers by a wider group of scientists.Whereas conventional flow cytometry detects fluorochromes’ emission peaks, spectral flow cytometry measures full fluorescence spectra from each particle and differentiates emission spectra shapes on a wide continuum of wavelengths.1
A major feature of spectral flow cytometry is, of course, spectral unmixing—that is, analysis of the spectral data to extract revealing information. Spectral unmixing can provide accurate estimates of label intensity and resolve fluorochromes with significant spectral overlap. The algorithm used for this process replaces the compensation matrices of conventional flow cytometry and treats autofluorescence as an independent parameter.1
All of this adds up to less-complex, more-capable instrumentation as well as greater flexibility, as researchers get much more choice in designing assays.
Commercial availability facilitates research
Though spectral cytometry was alive in the minds of innovators as early as the 1980s, it wasn’t until 20 years later that the availability of highly sensitive PMT arrays enabled researchers at Purdue University’s Cytometry Laboratories (West Lafayette, IN) to develop prototype systems.4 The Purdue team secured a U.S. patent on the design, which Sony Life Sciences subsequently licensed. In 2014, Sony introduced the first commercially available system, the SP6800 Spectral Analyzer. That same year saw the launch of a company called Cytek Biosciences (Fremont, CA), which soon released its Aurora spectral flow cytometer, followed in 2018 by a line of compact systems called Northern Lights, based on patent-pending technology (see Fig. 3).The availability of commercial systems has resulted in research that highlights the capabilities of spectral flow cytometry in situations where conventional flow cytometry struggles to deliver. For instance, a 2016 study demonstrated the ability of the Sony system for analysis of cell populations in tissues such as heart and intestine: The technique characterized 21 parameters (with 19 fluorophores), provided high-resolution analysis of specific populations, and effectively handled their autofluorescence—something conventional flow cytometry cannot do.1
A later study used spectral flow cytometry to investigate tumor/immune system dynamics using tissue samples from patients with non-small cell lung cancer (NSCLC).5 NSCLC, the most common lung cancer type, is typically not diagnosed until patients are at an advanced stage of the disease. While recent immunotherapy progress is promising for new treatment, researchers need to identify novel markers to understand it, and finding such markers requires appreciation of biological dynamics.
In their work to investigate NSCLC tissue specimens paired with blood samples for circulating tumor cells (CTCs), the researchers used Cytek’s Aurora to simultaneously profile multiple immune and cancer markers at the single-cell level. The findings revealed an association between high levels of both immune- and cancer-marker expressions with disease aggressiveness indicated by CTC volume. The researchers note that knowledge gained from these studies has boosted their understanding of the immune system, cancer cells, and interaction between the two—all of which promises to expedite patient diagnosis, disease monitoring, and drug discovery. The work may also facilitate successful precision cancer immunotherapy.
In fact, cancer research is helping to drive the demand for flow cytometry, which numerous market researchers concur will grow dramatically in the coming years. It seems that spectral flow cytometry is particularly suited for rapidly growing areas of cancer research, including immune profiling, which helps determine a patient’s expected reaction to treatment; chimeric antigen receptor (CAR) T-cell therapy, a novel treatment that uses altered white blood cells to target cancer; and immuno-oncology, which aims to assist the body’s own immune system in killing cancer.
Currently, immuno-oncology uses genomics technology to accomplish gene profiling of cells. But while genomics platforms can reveal a cell’s type and condition, it is challenged to perform with heterogeneous cell populations, so researchers will typically use a cell sorter before applying the genomics technology. Spectral flow cytometry, on the other hand, can quickly and objectively reveal the status of individual cells in a heterogenous population.
An emerging generation
“While the benefits offered by flow cytometry are well known throughout the scientific community, adoption has lagged due to its cost-prohibitive, workflow-intensive nature,” says Wenbin Jiang, Ph.D., CEO of Cytek Biosciences. “We’re on a mission to make the advanced instrumentation that was once available to very few scientists accessible to more researchers.”
To realize that mission, Cytek introduced its Northern Lights series of instruments, dramatically reducing the price of admission for spectral cytometry, and enabling capabilities particularly important for cancer research. Northern Lights can be upgraded from one laser (up to nine colors) to three (providing access to more than 24 colors). The systems let researchers extract from one sample the volume of information that normally requires three or four tubes of sample—which is significant in cancer research requiring tumor aspirates, which are available only in very small quantities.
Because Northern Lights uses a single optical configuration for all applications, along with relatively low-maintenance lasers (compared to the sometimes expensive, high maintenance lasers used in conventional flow cytometry), it tends to reduce experimental errors and save time. The company is also emphasizing ease of use with its systems and bolstering streamlined system design with support so that customers are fully able to use the systems’ capabilities.
Whereas most cytometers use PMTs to detect photons and convert them to electrons, the Cytek systems use miniature avalanche photodiodes (APDs) to handle this work. Thanks to their pencil-eraser size, the APDs contribute to system miniaturization, but they also boost sensitivity, with a high quantum efficiency (QE) in the 400–900 nm range. As such, they contribute two-thirds less noise than PMTs, and because noise can mask dim staining, they enable better resolution.
Jiang says that Cytek has a product roadmap to get spectral flow cytometry into the hands of more “envelope pushers.” What will the next innovations bring?
REFERENCES
1. S. Schmutz, M. Valente, A. Cumano, and S. Novault, PLoS ONE, 11, 8, e0159961 (2016).
2. J. P. Nolan and D. Condello, Curr. Protoc. Cytom., 63, 1, 1.27.1–1.27.13 (2013).
3. G. Grégori et. al., Curr. Top. Microbiol. Immunol., 377, 191–210 (2014).
4. See http://nolanlab.com/spectral-fc.html.
5. X. Wang et al., Proc. AACR Annual Meeting 2018, Chicago, IL (Apr. 14-18, 2018); doi:10.1158/1538-7445.am2018-2112.
About the Author

Barbara Gefvert
Editor-in-Chief, BioOptics World (2008-2020)
Barbara G. Gefvert has been a science and technology editor and writer since 1987, and served as editor in chief on multiple publications, including Sensors magazine for nearly a decade.