Medicalwatch: Optical-biopsy device nears commercial reality
A hand-held, noninvasive laser device that helps physicians decide whether a patient needs further testing for cancer is poised to become the first optical-biopsy system to gain US Food and Drug Administration (FDA) clearance. The Gastroenterology and Urology Devices Panel of the FDA has voted to recommend clearance of the virtual Biopsy System developed by SpectraScience (Minneapolis, MN), for use initially in colonoscopy and flexible sigmoidoscopy in the early detection of colon cancer.
At present, colorectal cancer is primarily diagnosed by the detection and analysis of polyps identified by inserting an endoscope into the colon. Large polyps are usually removed immediately and sent to pathology for identification. With small polyps, random representative biopsies can be taken to determine the next step, but often the screening physician will refer the patient for a colonoscopy directly, increasing health-care costs.
New technologies that improve the physician's diagnostic capabilities and allow for earlier treatment would likely increase survival rates, improve quality of life, and reduce costs. Studies indicate that diagnostic screening and appropriate treatment procedures could prevent 20% to 40% of potential colorectal cancers and up to 30% to 50% of colorectal-cancer deaths.
This is where the SpectraScience virtual Biopsy system comes in. The system uses laser-induced fluorescence (LIF) through an endoscope to make contact with the polyp, optically scan it, and provide an instant analysis. It is intended primarily for use with small polyps to aid clinicians in making diagnostic decisions as to which polyps should be biopsied, removed, or monitored over time for changes. It provides an instant analysis that can be immediately communicated to the patient, eliminating the several-day waiting period for pathology results from a conventional excisional biopsy.
Diagnostic accuracy
A multicenter study conducted by SpectraScience in support of the virtual-biopsy device suggests that the diagnostic accuracy of endoscopy for distinguishing between neoplastic and non-neoplastic colonic polyps is enhanced when combined with optical-biopsy measurements. The clinical trial revealed that physicians' diagnostic accuracy in identifying cancerous or precancerous polyps was 88.9%. Using the virtual Biopsy System increased that accuracy to 97.2%, according to the company.
During clinical trials, when a polyp was identified via colonoscopy, LIF measurements were made from the polyp and from surrounding, normal-appearing mucosa using an integrated optical-fiber-biopsy forceps system. The system made it possible to biopsy the precise locations from which the LIF measurement was made. Fluorescence excitation was produced at 337 nm using a pulsed nitrogen laser, and the fluorescence was collected over a wavelength range of 350 to 600 nm.
Fluorescence was recorded by placing a small optical fiber in contact with the site of interest and activating the system by depressing a foot pedal. The spectra were stored for further analysis. Biopsies were obtained from all measurement sites; the polyp was completely resected by standard methods and submitted for pathologic assessment.
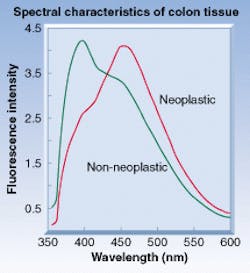
For comparison, polyps were classified as neoplastic or non-neoplastic, based on histology, the endoscopist's impression (endoscopy alone), and from endoscopy with the optical-biopsy system (see figure). In the last case, polyps were classified as neoplastic if either the endoscopist or the optical-biopsy system classified the polyp as neoplastic. The polyps were classified using a diagnosis algorithm generated from spectra derived from data obtained in a previous study. The diagnostic performance of the combined endoscopy/optical-biopsy system was compared to endoscopy alone using diagnosis based in histology as the gold standard.
One hundred and nine patients with polyps were enrolled in the SpectraScience multisite study, resulting in usable spectra from 165 polyps and 101 normal "control' areas. The polyps were classified by histology as neoplastic (110) or non-neoplastic (99). Broadband fluorescence was observed from all tissue sites when illuminated with 337-nm light. The fluorescence can be characterized by two predominant bands centered at 397 and 455 nm, respectively. The 455-nm band is more prevalent in the neoplastic group, while the 397-nm band is seen more often in the non-neoplastic group.
The results were cross-tabulated with clinical pathology. The endoscopist's impression combined with the optical-biopsy system was able to identify neoplastic lesions with sensitivity 97%, specificity 75%, negative predictive value 97%, and positive predictive value 74%. In general, the predictive accuracy of endoscopy with optical biopsies was better than prediction based on endoscopy alone. The increase in performance was most notable for polyps with diameters less than 5 mm.
The virtual Biopsy System is the first fully developed product from SpectraScience to incorporate the company's proprietary LIF technology. SpectraScience is pursuing a strategy of using this technology as an enabling platform for use in the detection of cancers of the prostate, cervix, bladder, and esophagus.
ACKNOWLEDGMENT
Additional reporting for this column was provided by Leslie Sabbagh, a freelance writer based in Cleveland, OH.
About the Author
Kathy Kincade
Contributing Editor
Kathy Kincade is the founding editor of BioOptics World and a veteran reporter on optical technologies for biomedicine. She also served as the editor-in-chief of DrBicuspid.com, a web portal for dental professionals.