Hyperspectral imagers shed light on pharmaceutical processing methods
The combination of a Food and Drug Administration (FDA) initiative promoting process analytical technology (PAT) and sizable financial risks and incentives in the pharmaceutical industry has created an opportunity for hyperspectral imaging technology that is being approached from a broadening array of spectral regions.
The PAT initiative essentially seeks to extend quality control in the pharmaceutical industry backward from finished product into manufacturing process using analytical technology, broadly defined to include chemical, physical, microbiological, mathematical, and risk-analysis methodologies. The FDA rationale is that “quality cannot be tested into products; it should be built in or should be by design.”1 Likewise, for pharmaceutical companies, the ability to reliably build or design effectiveness into medicines helps to ensure a steady stream of revenue from sales as well as patent royalties, while also helping to avoid tremendous losses (on the order of hundreds of millions of dollars) that can occur when a bad batch of drugs is not detected prior to reaching consumers.2
Hyperspectral imaging, specifically vibrational spectroscopy combined with two-dimensional visual imagery and referred to as chemical imaging, is contributing to PAT initiative efforts by helping to assess both the distribution of constituents and the molecular structure of solid-dosage pharmaceutical products such as capsules or tablets.
A typical pharmaceutical tablet is not just a pressed block of a single material, but a complex matrix containing active pharmaceutical ingredients (APIs), fillers, binders, disintegrants, lubricants, and other materials, according to Neil Lewis and colleagues at Spectral Dimensions (Olney, MD), a manufacturer of near-IR chemical imaging systems (see Fig. 1). So a relatively simple formulation with identical ingredients can produce widely varying therapeutic effects depending upon how the ingredients are distributed in the final matrix. Even when APIs can be formulated in very small dosages of 5 mg or less, practicality dictates that the finished tablet still be large enough for convenient handling, which requires uniform content distribution over a larger volume.
“Pharmaceutical makers also are developing advanced tablets for drug dosage management, which can provide longer, flatter, or sometimes complex bloodstream profiles,” Lewis wrote. “Approaches include the use of barrier layers, cored tablets, selective-release microspheres, and even osmotic pumps. These tablets essentially are highly engineered drug delivery systems in which the physical structure is as critical as the chemical composition.”3
In addition, the crystalline structure of molecules in a tablet or capsule can be just as important as physical structure and chemical composition in determining the effectiveness of a drug, particularly when a single constituent molecule can assume multiple crystalline structures or polymorphs. The predictable and reliable manufacture of stable polymorphs is not fully understood and involves as much art as science. Single-crystal x-ray diffraction gives the crystalline structure directly and is therefore the definitive method of determining the existence of a polymorph. Raman spectroscopy, however, offers a nondestructive and relatively convenient testing method that can be performed on solid tablets and capsules. It has begun to play an important role in screening crystalline salts and in pharmaceutical polymorph and hydrate transformation studies, according to Don Clark with Pfizer Global R&D (Kent, England).4
Raman and IR spectroscopy
In vibrational spectroscopy a sample is illuminated with incident radiation to excite molecular vibrations (see Fig. 2). Vibrations that change a molecule’s dipole moment can be detected using IR spectroscopy, and vibrations that change a molecule’s ability to be polarized can be detected with Raman spectroscopy. The techniques are often complementary because of transitions that are allowed in Raman but forbidden in the IR.5Mid-IR is the most commonly used form of vibrational spectroscopy because the spectral region contains all of the common vibrational energies of organic and nonmetallic inorganic species. But sample preparation for mid-IR spectroscopy requires a destructive grinding process and samples also must be free of water, which produces a prominent and obstructive background.
Chemical imaging
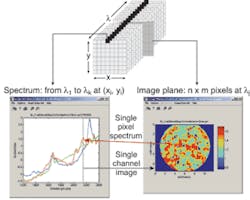
While vibrational spectroscopy can enable rapid compositional and structural determination as well as quantitation, spectroscopy alone cannot measure the spatial distribution and architecture of active ingredients. The relatively recent development of IR focal-plane arrays, however, has made it possible to combine vibrational spectroscopic information with direct imaging of the spatial distribution of the dosage components, thereby enabling direct observation of the chemical structure in near-real time (see Fig. 3).7The resulting chemical images provide visual representations in which false colors, created from Raman or near-IR spectroscopy, show the location of each chemical constituent in the sample. As mentioned earlier, each spectroscopy technique has strengths and weaknesses. For instance, Clark explained that Raman spectroscopy generally outperforms near-IR with inorganic compounds, while near-IR spectroscopy easily differentiates cellulose and “sugar-like” compounds that can be difficult to differentiate using Raman spectroscopy.
A chemical image-fusion (CIF) technique has been developed to exploit the complementary benefits of near-IR and Raman spectroscopy in which both Raman and near-IR microscopy can map exactly the same area of a sample. The chemical images from the two mapping techniques are combined, or fused, to produce a single composite image.
The fused chemical image is currently constructed using “simple” overlaid chemical images taken from individual Raman and near-IR mapping data sets, says Clark. However, the sophistication of the method is likely to improve as chemometric methods are developed to process both data sets simultaneously (see “Software combines chemometrics with image analysis,” p. 97).4
Terahertz pulsed spectroscopy
Infrared and Raman spectroscopy probe intramolecular vibrations. But a promising newcomer, terahertz pulsed spectroscopy, probes the intermolecular or phonon modes that characterize a crystalline structure, potentially offering a third vibrational spectroscopy window for nondestructively probing of the compositions and molecular structures of solid-dose pharmaceuticals. So far, the technique has demonstrated an ability to characterize and quantify solid-state forms of bulk APIs including polymorphs, and is being extended to investigate the API in tablets. A fast acquisition rate (obtaining data wave forms in less than 100 ms) indicates possible applicability to high-throughput polymorph screening. A commercial product has been introduced and several large pharmaceutical companies are exploring its potential.8
REFERENCES
- www.fda.gov/cder/OPS/PAT.htm
- A. Goho, Science News 166(8) 122 (Aug. 21, 2004).
- E. N. Lewis, J. Schoppelrei, E. Lee, Spectroscopy 19(4) 26 (April 2004).
- D. Clark, American Pharma. Rev. 7(4) 76.
- Kaiser Optical Systems (Ann Arbor, MI), Raman Products Technical Note 1101 [www.kosi.com/raman/resources/technotes/index.asp?view=TN1101]
- Kaiser Optical Systems (Ann Arbor, MI), Raman Products Technical Note 1102 [www.kosi.com/raman/resources/technotes/index.asp?view=TN1102]
- E. N. Lewis, J. E. Carroll, F. Clarke, Near-IR News 12(3) 16 (2001).
- Bruker Optics (Ettlingen, Germany), Application Note AF #502E, [www.brukeroptics.com/downloads/AF502E_TeraHertz-TPIspectra1000.pdf]
Software combines chemometrics with image analysis
A wide variety of chemical imaging systems based on Raman, ultraviolet, visible, near-infrared, mid-infrared, fluorescence, photoluminescence and other spectroscopic techniques have been developed for applications in a diverse range of manufacturing industries, such as pharmaceuticals, coatings and plastics, and semiconductors, in which product performance depends upon the spatial distribution of chemical constituents. Effective monitoring of high-throughput manufacturing processes in such cases requires simultaneous and rapid acquisition and processing of data from diverse sources.
Chemometrics (a statistical discipline within chemistry for extracting information from multivariate chemical data and optimizing analytical processes) has been combined with traditional digital image-analysis technology in commercially available software for precisely this purpose. The software follows a cyclical multistep analytical process that begins with image collection in a manner that accommodates the desired range of chemical image-acquisition configurations, spectroscopic techniques, spectrometers, imaging detectors, sampling sizes, and collection times-all ideally through one graphical user interface. The second step in the analysis cycle involves data preprocessing to minimize measuring instrument responses unrelated to the imaged sample. Once instrument noise has been suppressed, qualitative processing can find out what is present and how it is distributed.
Quantitative analysis then develops concentration-map images, and results obtained from preprocessing, qualitative analysis, and quantitative analysis can then be visualized. Traditional image analysis can then be applied, and the final step in the cycle, automation of key steps or of the entire chemical image-analysis process, might entail tabulating results according to preselected criteria such as particle size and shape or chemical composition. To help the user make intelligent decisions, the ideal analysis package should support the user’s efforts to carefully plan experiments and optimize instrument parameters and should allow the maximum amount of information to be extracted from chemical images.1
REFERENCE
- ChemImage (Pittsburgh, PA), “The Role of Chemometrics in Chemical Image Analysis” (November 2000). [www.chemimage.com/resources/abstracts/pdf/ The_Role_of_Chemometrics_in_Chemical_Image_Analysis.pdf]
About the Author
Hassaun A. Jones-Bey
Senior Editor and Freelance Writer
Hassaun A. Jones-Bey was a senior editor and then freelance writer for Laser Focus World.
![FIGURE 1. Pharmaceutical tablets have essentially become highly engineered drug delivery systems. From left to right: Oros, an Alza-developed tablet used by Pfizer and others, retains its shape in gastric fluids and releases therapeutic doses through an osmotic aperture; inorganic or polymeric microspheres selectively release adsorbed drugs with selected target therapies at staggered concentrations and release rates to provide a complex therapeutic pattern in the bloodstream; a barrier-layer reservoir capsule refreshes the drug concentration in the upper chamber as the drug releases to maintain constant concentrations and provide long drug-level plateaus in the bloodstream; and a cored tablet sequentially delivers one drug at two distinct concentrations or delivers two different drugs back-to-back [7]. FIGURE 1. Pharmaceutical tablets have essentially become highly engineered drug delivery systems. From left to right: Oros, an Alza-developed tablet used by Pfizer and others, retains its shape in gastric fluids and releases therapeutic doses through an osmotic aperture; inorganic or polymeric microspheres selectively release adsorbed drugs with selected target therapies at staggered concentrations and release rates to provide a complex therapeutic pattern in the bloodstream; a barrier-layer reservoir capsule refreshes the drug concentration in the upper chamber as the drug releases to maintain constant concentrations and provide long drug-level plateaus in the bloodstream; and a cored tablet sequentially delivers one drug at two distinct concentrations or delivers two different drugs back-to-back [7].](https://img.laserfocusworld.com/files/base/ebm/lfw/image/2016/01/th_165117.png?auto=format,compress&fit=fill&fill=blur&q=45&w=250&width=250)
![FIGURE 2. Imaging optics collect diffuse reflected near-IR radiation from the sample surface before it passes through a near-IR tunable filter and forms an image on an IR focal-plane-array detector. The filter continuously steps through a predetermined spectral interval and an image is stored at each wavelength. Isys software provides image analysis [7]. FIGURE 2. Imaging optics collect diffuse reflected near-IR radiation from the sample surface before it passes through a near-IR tunable filter and forms an image on an IR focal-plane-array detector. The filter continuously steps through a predetermined spectral interval and an image is stored at each wavelength. Isys software provides image analysis [7].](https://img.laserfocusworld.com/files/base/ebm/lfw/image/2016/01/th_165116.png?auto=format,compress&fit=max&q=45&w=250&width=250)