SPECTROSCOPY: LIBS comes on strong
The last ten years have seen a revival in laser-induced breakdown spectroscopy. Its simplicity and ability to excite multiple sample types are dramatically increasing its applications.
STEVEN G. BUCKLEY
Recent years have seen a proliferation of exciting developments in laser sources, detectors, and optical instrumentation. In particular, compact and powerful lasers are now available that can provide hundreds of millijoules of pulse energy in approximately 6- to 10-ns pulses. These new, increasingly portable sources have opened up a host of new applications in laser-induced breakdown spectroscopy (LIBS), a useful analytical tool.
Laser-induced plasmas were reported as early as 1962, shortly after the discovery of the ruby laser, and they enjoyed some attention in the 1960s as an analysis technique. More recently, the work of Leon Radziemski and David Cremers in the 1980s is credited with launching LIBS as a stand-alone analytical method.1
The LIBS process
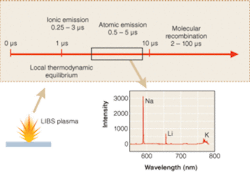
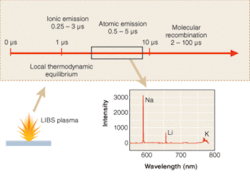
Laser-induced breakdown spectroscopy utilizes a focused pulse from a high-peak-power laser to create a plasma in a solid, liquid, or gaseous media. Some of the energy in the plasma is used to ablate solid or liquid material (if present), and the plasma rapidly expands, sending a shock wave into the surrounding media. In the core of the plasma, effective temperatures can easily exceed 50,000 K or several electron volts. During this stage, material in the core of the plasma is vaporized and atomized, and the plasma is typically highly ionized. Depending on conditions, but usually after 0.5 to 1.0 µs, the neutral species in the plasma typically reach local thermodynamic equilibrium. From this time onward, upper electronic states of atoms are thermally populated in Boltzmann equilibrium, such that the emission intensity, I, of the atomic fluorescence, is expressed as I ≅ exp(-E/kT), where E is the upper-state energy level of the fluorescing species and k is the Boltzmann constant.
As the plasma cools, continuum emission from the plasma (Bremsstrahlung emission) fades, typically much faster than emission lines from neutral and singly-ionized atomic lines, such that each elemental emission line has a particular optimum in a particular plasma.2 This optimum depends on the time/temperature history of the plasma, which in turn is dependent on the laser pulse energy and pulse length, among other things. For typical 200- to 300-mJ pulses, with approximately 10‑ns pulse duration, a particular sequence of events occurs in the plasma (see Fig. 1). In higher-energy, longer-pulse plasmas, events are shifted to longer times, while for lower energy, shorter-pulse plasmas, events are shifted to shorter times.
Configurations
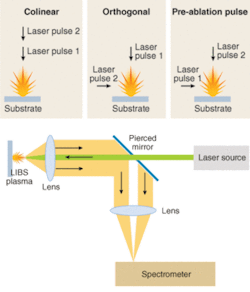
Laser plasmas are well-known ablation sources, and researchers in laser-ablation mass spectrometry, and recently in LIBS, have been working on understanding ablation-plume dynamics on solid samples. The fact that the plasma plume is a hydrodynamically and chemically evolving structure leads naturally to the idea that there are different, and potentially optimal, experimental configurations for particular situations.
In particular, researchers have investigated the merits of “double-pulse” LIBS, in which two laser pulses are used with very short (0.1 to 5 µs) interpulse spacing. Double-pulse LIBS can be performed on a solid target with several geometries (see Fig. 2). In the simplest case, one pulse follows another orthogonally onto the surface to be sampled. The second pulse is largely intercepted by the ablation plume from the first pulse, and the interaction further excites the expanding plume, significantly enhancing emission. The second pulse can also be orthogonal to the first, such that it clearly will not impact the surface but will still excite the expanding plume. In a third configuration, the first pulse can be in the air above the sample surface. Such a “preionization” pulse also dramatically enhances the atomic emission signal.
The choice of optical collection and detection is also a key element of a LIBS system. Some applications utilize bulk optics, such as 90°-detection methods or detection of the light propagating backward toward the detector, which is particularly useful when there is only a single optical access. In other situations, direct fiber-based collection is very successful, particularly in settings in which there is a fixed target. Even with bulk-optic light collection, in most cases the light is eventually coupled into a fiber for dispersion in a spectrometer.
Traditional Czerny-Turner spectrometers are the workhorse of most spectroscopists and these are still most commonly used for LIBS. However, miniature, portable, fixed-grating, fiber-coupled CCD or photodiode-array detectors such as those from StellarNet (Tampa, FL) and Ocean Optics (Dunedin, FL) have made significant inroads into the LIBS community and will continue to do so as gate detection with these spectrometers improves. These detectors have the advantage of low inherent noise and simple operation. In addition, echelle spectrometers, such as those from Catalina Scientific (Tucson, AZ) and Andor (Belfast, U.K.) are frequently coupled with intensified CCD cameras to provide wide wavelength coverage (about 200 to 700 nm).
Such broadband systems, or broadband miniature systems with CCD detectors, have the unique ability to measure associations between elements that can be widely spectrally separated in a single laser shot. If there is a need for high time resolution, the gated, intensified CCD camera remains the best choice for a detector, while for less demanding applications the time gating built into the newer, portable spectrometers may suffice.
PharmaLaser (Boucherville, Quebec, Canada), XRF Scientific (Perth, Australia), Marwan Technology (Pisa, Italy), and Ocean Optics, among others, have designed dedicated analytical systems, including quantification software, for LIBS. Several companies, including Ocean Optics and StellarNet, have recently come out with portable LIBS systems for mobile applications.
Example applications
The relative simplicity of an LIBS system-a pulsed laser and a detector plus focusing and collection optics-and the ability of LIBS to excite multiple sample types, potentially with little or no sample preparation, have fostered a diverse array of applications. Able to detect as little as 1 to 10 femtograms of many elements, LIBS provides ample sensitivity for many demanding measurements.
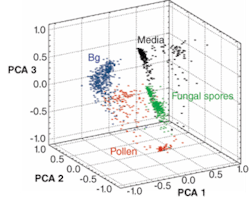
One of the most exciting applications has been in the area of forensics and homeland security. Distinct elemental signatures may hold promise for the detection of biological agents and improvised explosive devices in the battlefield.3, 4 Particles, spores, and contaminant spectra from surfaces can be effectively discriminated using unique elemental fingerprints, often correlated using a chemometric or statistical method such as principal components analysis. For example, the discrimination of individual spores of pollen, fungus, bacterial growth media, and anthrax simulant bacillus globigii (BG) can be achieved using three-axis principal component analysis with 30 features from broadband spectra (see Fig. 3).
Rapid scanning and screening of material is another key application for LIBS. Researchers such as Reinhard Noll and his group from the Fraunhofer Institute of Laser Technology in Aachen, Germany, are developing high-speed methods of discriminating materials and of interrogating metal samples for potentially harmful impurities. They have developed a system that rapidly detects metals and metal alloys on a conveyer and sorts them into bins; another solution “maps” elemental concentrations on a surface to determine associations between elements and detect trace impurities.
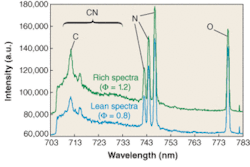
Laser-induced breakdown spectroscopy has also had significant application in energy-related measurements. From up-front measurement of coal composition, as pioneered by David Ottesen and coworkers at Sandia National Laboratories (Albuquerque, NM), to recent measurements in flames and in engines, to combustion process emissions, LIBS has been a useful tool in the harsh and complex environment of practical combustion devices (see Fig. 4). Spectral peaks related to carbon (C), nitrogen (N), and oxygen (O) are shown, and ratios of these peaks (C/N or C/(N+O)) are directly related to the air/fuel ratio. The application of this technique in an engine or another combustion device can reveal a great deal about combustion physics and potential emissions and operation parameters.
The future of LIBS
Numerous other LIBS applications are being developed with much promise, in addition to hybrid techniques such as LIBS/Raman and LIBS/laser-induced fluorescence. A key to the future of these techniques is the quantification of the spectra that result. Significant progress has been made in this regard, but additional work remains if LIBS is to be on an equal diagnostic footing with methods such as inductively coupled plasma (ICP) atomic-emission spectroscopy, and other accepted analysis techniques. With the advantages of simplicity and little or no sample preparation requirement, there is a strong incentive to continue the development of LIBS technologies.❏
REFERENCES
1. D.A. Cremers and L.J. Radziemski, Analytical Chemistry 55(8) (1983).
2. B. Fisher, H. Johnsen, S. Buckley, Applied Spectroscopy55, 10, 1312 (2001).
3. J. Hybl, G.A. Lithgow, and S.G. Buckley, Applied Spectroscopy 57, 10, 1207 (2003).
4. C. López-Moreno et al., J. Analytical Atomic Spectrometry (Advance article online), DOI: 10.1039/b508055j (2006).
5. F. Ferioli, P.V. Puzinauskas, and S.G. Buckley, Applied Spectroscopy57(9) 1183 (2003).
STEVEN G. BUCKLEY is an associate professor at the Center for Energy Research in the Department of Mechanical and Aerospace Engineering at the University of California, San Diego, 9500 Gilman Drive, MS 0411, La Jolla, CA 92093-0411; e-mail: [email protected].