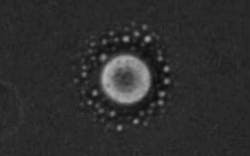
Scientists at the University of Glasgow (Glasgow, Scotland) have managed to separate two liquids in a mixture using a low-power laser-diode beam, a technique that they claim will lead to new ways of manipulating matter and creating crystals for industry.
In the new approach, laser tweezing (light-induced mechanical forces that push on matter) is used to harness fluctuations associated with a so-called critical point and to drive the system towards a phase-separated state. The approach was experimentally demonstrated using a simple liquid mixture. Although the experiment was done on liquids, the technique is aimed at the crystallization process.
The faithful production of crystals is critically important in science and technology as crystals are used in computer chips, drugs, paints, LEDs, photovoltaic cells, and so on. However, producing the right type of crystal is critical. We currently lack the ability to fully control the crystallization process and this can lead to extremely costly problems in industry.
"In our experiments, we used a simple mixture of two liquids and a relatively low-power laser diode to suck one of the liquids out of the mixture," says Klaas Wynne, who designed and developed the approach. "So it's a little bit like making a cup of tea, stirring in some milk, and then using a laser to suck the milk out again. It may seem really counterintuitive, but it's all within the laws of physics."
"These are the first steps towards a full understanding of the role that critical fluctuations play in crystal nucleation," adds Finlay Walton, who carried out the work. "Our aim is to gain full control over nucleation, including the type of crystal that is produced."
Source: https://www.gla.ac.uk/news/headline_573407_en.html
REFERENCE:
1. Finlay Walton and Klaas Wynne, Nature Chemistry (2018): doi: 10.1038/s41557-018-0009-8.
About the Author
John Wallace
Senior Technical Editor (1998-2022)
John Wallace was with Laser Focus World for nearly 25 years, retiring in late June 2022. He obtained a bachelor's degree in mechanical engineering and physics at Rutgers University and a master's in optical engineering at the University of Rochester. Before becoming an editor, John worked as an engineer at RCA, Exxon, Eastman Kodak, and GCA Corporation.