ADVANCED SPECTROSCOPY: Attosecond spectroscopy moves beyond the atomic scale
In his December 2008 Annual Technology Review, John Wallace, Laser Focus World senior editor, noted an important advance in attosecond science. "One of the highlights this year was the creation of 80-attosecond pulses of light," Wallace wrote, in describing the achievement of researchers at the Max-Planck-Institut für Quantenoptik (MPQ) and the Ludwig-Maxmilians-Universität (both in Garching, Germany).
The application of these 80 as pulses to the field of spectroscopy is vast. "For example, in life sciences attosecond spectroscopy may ultimately create ways of understanding the microscopic origins of how a disease, such as cancer, emerges and develops at the most fundamental level: in terms of the motion of electrons," said Ferenc Krausz, director of MPQ, head of the Attosecond and High Field Physics division of MPQ, and pioneer of femtosecond and attosecond science.1 Today, the attosecond science breakthrough by MPQ and partner institutions is leading to important real-world ultrafast spectroscopy applications that seek to understand the very stuff that matter and life is made of.
Building an attosecond spectrometer
To create an attosecond light pulse, a phase-stabilized femtosecond laser with pulse-to-pulse uniformity in terms of intensity, frequency, and physical shape are input to a gas jet such as neon. The femtosecond pulse pulls electrons from the neon atoms (field ionization), which then collide with their "parent" atoms in a recombination process and produce a synchronized, attosecond duration, extreme ultraviolet (XUV) pulse in a high-order harmonic generation (HHG) process.
The most straightforward method of investigating ultrafast processes on the atomic scale is pump-probe spectroscopy: a system under test is excited with an attosecond "pump" pulse and properties of the system (absorption, photoelectron or secondary electron energy) are measured as a function of the delay of a second "probe" pulse. "Ultrafast phenomena can be measured by taking snapshots of electron energy distributions at different time delays with respect to the event that triggers the process," says Reinhard Kienberger, a professor at Technische Universität München (Munich, Germany) and a researcher at MPQ. "Consequently, an attosecond spectrometer would include, for example, an energy (momentum) resolving detector for electrons (or ions), and a delay unit for pump and probe pulses, and there are many different forms of this combination."
Understanding ionization dynamics
When the outermost electrons are ripped from atoms in the process of ionization, whether by a strong laser pulse or through high-energy interactions that occur everyday in the center of the Earth and Sun, in the upper atmosphere, or within our own bodies, the ionized atoms are left behind to recombine with free electrons and revert to their original form, or to combine with other oppositely charged ionized atoms to create specific molecules. Understanding these ionization processes is, in effect, a means of understanding just how physical and chemical processes occur in the universe. "Any chemical or biochemical reaction is first started by the movement of electrons," says Kienberger.
At MPQ, Kienberger is working to understand the electron dynamics of solids, with an eye toward improvements in communications networks.2
By pumping a solid with a high-energy (100 eV) attosecond pulse and using the linearly polarized electric field of a probe laser (750 to 800 nm), electrons ejected from the solid feel the probe laser field and are deflected in the direction of polarization at different instances in time after they travel several angstroms to get to the surface of the solid. By measuring the electric-field distribution of these electrons at different time delays, a spectrogram can be created that paints a picture of the temporal behavior of electrons for different solid materials.
"In the 1960s, scientists studying semiconductors didn't know that their work would result in today's laptops and cell phones. In a similar way, it is impossible to predict how attosecond science will influence our world 20 years from now," says Kienberger. "For example, the collective motion of electrons within a wire or conductor limits the speed that the electrons can go. If you can imagine nanostructures that could channel the highest energy single electrons, speeding up a communications network by a factor of 100,000 could be possible."
As head of research at the Institute for Quantum Electronics at the Swiss Federal Institute of Technology (ETH Zurich, Switzerland), Ursula Keller and her group are also studying high-energy ionization dynamics, and recently used attosecond angular streaking to place an intensity-averaged upper limit of 12 as on the tunneling delay time (the time it takes an electron to escape the binding potential of an atom before the atom becomes ionized) in the strong-field ionization of a helium atom—the fastest process that has been measured to date.3, 4 Previous theoretical work by other groups had yielded values of 450 to 560 as.5, 6
The physical setup for the experiment uses what Keller calls an "attoclock" based on an almost circularly polarized infrared (IR) 725 nm laser pulse from a Ti:sapphire laser focused onto helium atoms in a target recoil ion momentum spectroscopy (COLTRIMS) apparatus. "In this device, the laser pulses move in a circle in space rather than in the form of a wave as is normally the case with a light beam," says Keller. "The electric field of the IR source rotates once through 360° in space over 2.4 fs, creating a kind of clock face with an attosecond pointer instead of a second hand. The length of the IR pulse is only about 5 fs, which means that the laser-induced tunneling ionization takes place in principle within one revolution of the pointer. The COLTRIMS detector then measures the exact direction in which the electron exits the atom and therefore the time in the clock face of this attoclock."
How do electrons move?
Like Keller, researchers at Lawrence Berkeley National Laboratory (LBNL) and the University of California, Berkeley (UCB), are using attosecond spectroscopy to understand not only "how fast" electrons move, but just "how" electrons move; in other words, the direction they move and why. "Our work on carrier-envelope-phase or CEP of few-cycle laser pulses shows that the electric-field response of electrons is determined by the nature of the physical laser field and not just the intensity envelope of the laser pulses," says Mark Abel, graduate student at UCB and guest researcher at LBNL.7
Abel and his colleagues have shown how CEP—the temporal offset between the laser cycle maximum and the pulse envelope maximum, converted to a phase using the laser frequency—affects the angular distribution of photoelectrons generated using relatively weak laser pulses (two orders of magnitude less than the fields required for tunnel ionization, or around 1013 W/cm2) in the multiphoton regime (see also "Single-shot technique measures CEP for few-cycle pulses."
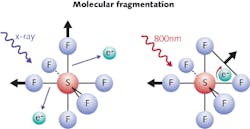
Abel's laser pulses target the weaker binding forces between atoms. "We are currently working on attosecond spectroscopy applications to chemical dynamics," he says. "Specifically, we are interested in attosecond timescale couplings between excited-state electronic surfaces that can cause the fragmentation dynamics of polyatomic molecules to change" (see figure).
Abel says that an understanding of electron dynamics in these low-energy processes could shed light on how human eyes detect photons and how photons are converted to energy in photosynthesis, for example. "Understanding how electrons move inside of and between molecules is important to eventually understanding the efficiency of these natural processes," he says.
Whoaaaaa—not so fast!
"In a quantum system, by taking a 'look' at the object, the object is destroyed," says Chii-Dong Lin, university distinguished professor at Kansas State University (KSU; Manhattan, KS). "Due to the energy-time uncertainty relationship (similar to the Heisenberg uncertainty relationship in quantum mechanics), the 'energy levels' of an object are no longer a useful concept. The attosecond pulses available today can be used as a pump to initiate a time-dependent quantum system, but they are not intense enough to be used as a probe and therefore create a fuzzy molecular movie."
To increase the power level of attosecond pulses, professor Zenghu Chang and his group at KSU are creating single isolated attosecond pulses using a double optical gating or "DOG" scheme.8 Their approach allows the usage of relatively long-pulsed (30 fs) lasers, which are much easier to operate than 5 fs lasers and more easily scaled up in power. The group's CEP locking technique is being transferred to commercial products by Femtolasers (Vienna, Austria). Lin explains that his role as a theoretician is to provide some guidance for experimentalists like Chang such that more informative molecular movies can be created in the future.
REFERENCES
1. Nadya Anscombe, "Attosecond analysis," Nature Photonics Tech. Focus, Spectroscopy 2, p. 548 (September 2008).
2. R. Kienberger, Photonics Spectra 42(4) p. 68 (2008).
3. P. Eckle et al., Science 322(5907) p. 1525 (Dec. 5, 2008).
4. P. Eckle et al., Nature Physics 4, p. 565 (2008).
5. M. Büttiker and R. Landauer, Phys. Rev. Lett. 49, p. 1739 (1982).
6. L. V. Keldysh, Soviet Physics J. Experimental and Theoretical Physics (JETP) 20, p. 1307 (1965).
7. M. J. Abel et al., J. Physics B: Atomic, Molecular and Optical Physics 42, p. 075601 (2009).
8. H. Mashiko et al., Physics Rev. Lett. 100, p. 103906 (2008).
About the Author

Gail Overton
Senior Editor (2004-2020)
Gail has more than 30 years of engineering, marketing, product management, and editorial experience in the photonics and optical communications industry. Before joining the staff at Laser Focus World in 2004, she held many product management and product marketing roles in the fiber-optics industry, most notably at Hughes (El Segundo, CA), GTE Labs (Waltham, MA), Corning (Corning, NY), Photon Kinetics (Beaverton, OR), and Newport Corporation (Irvine, CA). During her marketing career, Gail published articles in WDM Solutions and Sensors magazine and traveled internationally to conduct product and sales training. Gail received her BS degree in physics, with an emphasis in optics, from San Diego State University in San Diego, CA in May 1986.