Kishan Dholakia and others at St. Andrews University (Fife, Scotland) have modified optical-tweezer techniques to provide rotational control of objects held in laser beams.1 Applications range from improved biological and chemical testing of trapped objects to transferring power to micromachines.
Optical-tweezer techniques have been developed over the past decade to hold microscopic objects in place using a focused laser beam, and are now established tools for biology, including inserting genes into cells or assisting with in vitro fertilization. The new method offers more control for similar applications. It allows researchers to control the speed of rotation and the angular distance through which the trapped particle moves. The researchers have demonstrated the technique on micrometer-sized objects, including a glass bead, a glass rod, and a hamster chromosome.
The technique also could be used to rotate micrometer-sized machinery. For example, Dholakia said, "Our technique could be used to drive motors, mixers, centrifuges, and other rotating parts."
The new technique is a modification of the optical-tweezers method, which traps an object of higher refractive index than its surroundings near the focus of a laser beam. In both cases, a particle gets trapped in the path of a light beam because some of the light refracts when it hits the object. This changes the momentum of the light and the momentum of the object, which gets steered toward the focus, where the light is most intense.
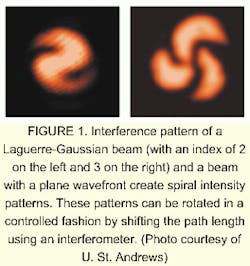
The St. Andrews work uses two beams rather than one. One of the beams has a simple plane wavefront. The other beam is a Laguerre-Gaussian (LG) beam. These beams, unlike typical Gaussian beams (which are circularly symmetric and with intensity cross sections that appear as a single bright spot with smooth edges), tend to transverse intensity cross-sections that look like concentric rings. They also are characterized by a helical wavefront. The Poynting vector in such a beam "corkscrews" as the beam propagates.
An analogy to the beam is a rope made of a number of twisted cords. If the rope is cut and viewed head-on, it shows 2, 3, or more cords. The position of the cords will differ depending on where the rope is cut, but the number of cords will remain the same, and the pattern of where the specific cords are located will repeat after some interval. If there are two cords, then the pattern and location of the cords repeats itself twice before each cord has made a complete circuit. If there are three cords, the pattern repeats itself three times before cords come back to their starting spots. In a similar way, the LG beam has an azimuthal index, which describes whether the beam's phase-front is a double- or triple- (or more) start helix.
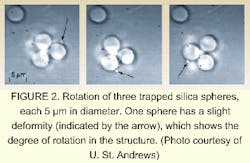
When coincident with the plane-wavefront beam, the interference pattern between the two creates a spiral intensity pattern (see Fig. 1). By changing the path length of one beam through a full period, the researchers are able to rotate the intensity pattern in a controlled fashion around the axis (see Fig. 2).Two other techniques have been developed for rotating objects, but they are neither as gentle nor as widely applicable as the St. Andrews technique.
Dholakia's group used a neodymium-doped vanadate laser as the laser source, and created the LG beam using a computer-generated hologram. The group trapped two 1-µm-diameter silica spheres and spun them at a rate of 7 Hz. The minimum optical power required to rotate the spheres was 1 mW. Because DNA can be bound to these spheres (when properly treated), this experiment could be extended to orienting DNA strands.
In another experiment, a glass rod was rotated, effectively creating an all-optical micro-stirrer. This could be used for stirring tiny volumes of liquids or for rotating parts of micromachines. And in a third demonstration, the researchers rotated a Chinese hamster chromosome without damaging it. Re-orienting chromosomes can be useful in biotechnology research, such as excising sections for use in polymerase chain reactions. The ability to rotate structures inside a cell, such as the mitochondria or cytoskeletal structures, can allow scientists to study these structures in more detail.
REFERENCE
- L. Paterson et. al., Science 292, 912 (May 4, 2001).