Nanoscale plasmonics begins to unravel Alzheimer’s disease
An optical sensor based on localized surface-plasmon-resonance spectroscopy is designed to detect a variety of ligands, including a biomarker for Alzheimer’s disease that is crucial in the development of an Alzheimer’s clinical diagnostic test.
The advent of the “nanotechnology” era in scientific research has ushered in the development of a multitude of devices and processes on the nanometer-length scale. One of the most prominent of these is biological sensors for the diagnosis of diseases, detection of environmental toxins, and drug discovery. The applications of these nanosensors are promising and wide-ranging; however, advances in technology because of nanoscale phenomena will only be optimized and fully used once the chemical and physical properties of the relevant materials are more thoroughly characterized and understood.
In 2001, a paper first described the response of the optical properties (or color) of nanoparticles to adsorbates via shifts in the extinction maxima of the localized surface-plasmon resonance (LSPR) of homogeneous silver nanoparticles fabricated using nanosphere lithography.1 This paper pointed the way to the development of a new class of nanoscale optical sensors (see Laser Focus World, May 2003, p. 153). A biosensor based on model systems demonstrated that, by designing the correct surface chemistry, highly quantitative and specific responses to biological analytes could be monitored by measuring optical changes. Significantly, the magnitude of the response of this sensor was more than an order of magnitude larger than expected. In addition, the LSPR approach to refractive-index sensing demonstrated the implications of “nanosizing” the dimensions of the widely used and commercialized propagating surface-plasmon-resonance (SPR) sensor.
Refractive-index detection
Propagating surface-plasmon resonances are evanescent electromagnetic waves that travel along the interface between a flat metal and dielectric, and arise from oscillations of the conduction electrons in the metal. Surface-plasmon-resonance sensors detect the local (that is, up to 1 µm away from the metal surface) refractive-index changes that occur when a target analyte binds to the metal film. These sensors have an inherent advantage over optical biosensors that require a chromophoric group or other label to transduce the binding event, in that the sensor detects the refractive index of matter itself. While the technique is totally general, if designed properly, specificity of the sensor can be incorporated via novel surface chemistries.
A novel nanoscale development, termed the LSPR nanosensor, is a refractive-index-based sensing device that relies on the extraordinary optical properties of noble-metal nanoparticles, including silver (Ag), gold (Au), and copper (Cu). The LSPR of noble-metal nanoparticles arises when electromagnetic radiation induces a collective oscillation of the conduction electrons of the individual nanoparticles, and has the consequences of wavelength-selective absorption, resonant Rayleigh scattering, and enhancement of local electromagnetic fields near the surface of the nanoparticle.
The development of biosensors is a crucial asset in the field of medical diagnosis. Traditional assays often fail by giving misleading false positives or negatives. In some cases, the assays are not sensitive enough to provide details at relevant concentrations. The success of the LSPR nanosensor (with the biotin/streptavidin and biotin/anti-biotin models) has led to the exploration of sensor response to other, pathologically relevant systems.
Since initial studies on model systems, LSPR nanosensor technology has advanced to allow the detection and analysis of Alzheimer’s disease (AD) and other complex biological systems. Several accomplishments have made this possible: the electromagnetic fields surrounding the nanoparticles for a sandwich assay have been optimized, nanoparticle adhesion has been improved without compromising nonspecific binding of biomolecules, and, finally, ADDLs/anti-ADDL technology has matured.
Electromagnetic-field control
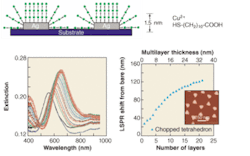
To best detect analytes using a “sandwich-assay” format, the electromagnetic fields that surround the nanoparticles must be optimized in a manner that balances the desired response from the target analyte and second antibody while minimizing nonspecific responses. Both of these properties rely on the control and understanding of the surrounding electromagnetic fields. This was accomplished by probing the long-range distance dependence of the LSPR of surface-confined noble-metal nanoparticles.2 It was suspected that the linear distance dependence found in straight-chain alkanethiol self-assembled-monolayer (SAM) formation was the thin-shell limit of longer-range, nonlinear dependence. To verify this, multilayer SAM shells based on the interaction of 11-mercaptoundecanoic acid and Cu2+ were assembled onto surface-confined noble-metal nanoparticles and were monitored using UV-visible spectroscopy (see Fig. 1).
FIGURE 1. Electromagnetic-field decay length is determined using nanoparticles. Multilayer binding chemistry is schematically depicted (top). Long-range LSPR spectroscopy of silver nanoparticles (width of 100 nm, height of 50 nm) was carried out for 0 through 20 layers of Cu2+/HS-(CH2)10COOH (lower left). The LSPR shift versus the number of SAM layers and layer thicknesses was measured for triangular silver nanoparticles (lower right). The inset contains a representative AFM image of the silver nanoparticles.
Measurement of the LSPR extinction-peak shift versus the number of layers and adsorbate thickness is nonlinear and has a sensing range that is dependent on the composition, shape, in-plane width, and out-of-plane height of the nanoparticles. Theoretical calculations based on an accurate electrodynamics description of the metal nanoparticle plus surrounding layered material indicate plasmon-resonance wavelength shifts that are in excellent agreement with the measurements. The calculations show that the sensing range is determined by the falloff of the average electric field around the nanoparticle. These studies determined that silver nanoparticles with in-plane widths of about 90 nm and heights of 25 nm produced electromagnetic fields that extend to about 35 nm away from the nanoparticle surface. This sensing distance proved to be the optimal distance for the desired sandwich-assay format.
Integration of AD biochemistry and the sensor
Although Alzheimer’s disease was discovered a century ago by Alois Alzheimer, a comprehensive understanding of the molecular basis of the disease has not yet been reached. One theory rests upon the widely documented amyloid cascade hypothesis, in which monomers of the amyloid beta (Aβ) protein aggregate into insoluble fibrils and deposit as plaques in the brains of AD sufferers. While it was traditionally believed that these insoluble forms of Aβ were the cause of neuronal degeneration, a debate has emerged as to whether the plaques are the cause of AD brain damage or simply evidence of another degenerative process.
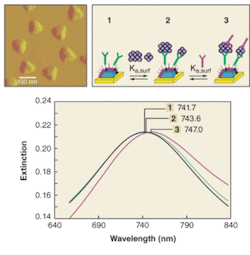
FIGURE 2. An LSPR biosensor for the detection of ADDLs is designed using a sandwich assay. First, surface-confined Ag nanoparticles are synthesized using nanosphere lithography on mica (upper left). Next, a SAM layer consisting of a mixed monolayer of OT and MUA passivates the nanoparticles for nonspecific binding and activates the nanoparticles for the attachment of the first anti-ADDL antibody, respectively (upper right). The first anti-ADDL antibody is covalently attached to the nanoparticles (step 1); samples are then incubated in varying concentrations of ADDLs (step 2); and, finally, to enhance the LSPR shift response of the ADDLs, the samples are incubated in a second, anti-ADDL (step 3). LSPR spectra were taken for each step of the sandwich assay for 100-fM ADDL (bottom). The spectra are for Ag nanoparticles after modification with 100-nM anti-ADDL antibody (λmax = 741.7 nm), 100-fM ADDL (λmax = 743.6 nm), and 100-nM anti-ADDL (λmax = 747.0 nm). All spectra were collected in nitrogen.
Significant evidence exists suggesting that a soluble form of Aβ, termed amyloid-derived diffusible ligands (ADDLs), is an equally potent neurotoxin. Based on this evidence, the LSPR nanosensor was designed to perform a “sandwich assay” for ADDLs (see Fig. 2). By monitoring the magnitude of the extinction-maximum shifts in response to varying ADDL concentrations on anti-ADDL functionalized nanoparticles, a surface-confined binding constant of 4.5 × 1010 M-1 was measured.3 The second antibody response revealed three different binding regimes for the sandwich assay. Concentrations of ADDL between 10 pM and 10 nM exhibit a strong binding constant for anti-ADDL of 9.5 × 108 M-1, while ADDL concentrations below 10 pM exhibit an even stronger antibody interaction with a binding constant of 7.3 × 1012 M-1. This places the limit of detection of the LSPR nanosensor at sub-100-fM ADDL concentrations, providing a detection capability well within the biologically relevant range.
Elimination of nonspecific binding
Ultimately, the LSPR nanosensor should be viable as a medical diagnostic tool. This requires that the sensor be extremely specific for its intended biotarget so that complex biological samples can be analyzed. When studies of the usefulness of the sensor as an AD diagnostic were first begun, the LSPR response was dominated by nonspecific interactions at low ADDL concentrations. Careful analysis revealed that the cause of this nonspecific response was a nanoparticle chromium (Cr) adhesion layer that interacted strongly with biomolecules.
In recent experiments, switching from glass to mica substrates has eliminated this effect. Because of its atomically flat surface, the mica provides a strong adhesion surface for Ag nanoparticles, rendering the use of a Cr adhesion layer unnecessary. Subsequent nonspecific binding studies testing the response of the LSPR nanosensor to anti-ADDL antibody in the absence of ADDLs revealed a zero response. Furthermore, testing the response of antibody-functionalized nanoparticles to the synthetic ADDLs vehicle yielded an irrelevant blue shift of less than 1 nm. All these results indicated that the LSPR nanosensor is capable of acting as an accurate diagnostic device for Alzheimer’s disease.
Preliminary human tests
After obtaining promising results from the nonspecific binding studies and demonstrating the quantitative capacity of the LSPR nanosensor using the sandwich assay, the sensor was tested as a detection device for actual biological samples. The nanosensor response to human brain extract and cerebrospinal fluid from both AD and control patients was analyzed using the same sandwich format used for synthetic ADDLs. In the case of human brain extract, the sensor responded with an overall shift of 10.7 nm for the diseased patient, compared with only a 0.6-nm shift for the control. However, because human brain extract can only be obtained post-mortem, the results for cerebrospinal fluid (CSF, which can be obtained from living patients) are more compelling. Studies comparing cerebrospinal fluid from AD and control patients revealed dramatic differences. While the control sample induced a shift of only 7.2 nm, CSF from the Alzheimer’s patient caused a response of 33.9 nm.
Outlook
The use of nanoparticles for the highly sensitive and selective detection of biomolecules has been demonstrated with model systems and complex human samples. The next challenge for the development of the LSPR nanosensor is to achieve analyte-multiplexing capability in an array format and integrate this into a microfluidic chip. By building from the already established (and commercially available) SPR sensing device, this is foreseeable in the near future.
While it is important to note that this technology for clinical diagnostics for Alzheimer’s disease is in its infancy, this work does demonstrate that LSPR nanotechnology offers new information not attainable with standard assay techniques. Future studies will continue to analyze these complex biological samples for AD and other diseases. Possible microfluidic implementation and rigorous sample testing of diseased and control populations will ultimately determine the success of the LSPR nanosensor.
ACKNOWLEDGMENT
The authors acknowledge support of the Nanoscale Science and Engineering Initiative of the National Science Foundation under NSF Award EEC-0118025. Any opinions, findings, and conclusions, or recommendations expressed in this material are those of the authors and do not necessarily reflect those of the National Science Foundation. We also thank Lei Chang, William Klein, George Schatz, and Shengli Zou for their experimental and theoretical assistance, materials, and expertise.
REFERENCES
1. M. D. Malinsky et al., J. American Chem. Soc. 123, 1471 (2001).
2. A. J. Haes et al., J. Phys. Chem. B 108, 109 (2004).
3. A. J. Haes et al., J. American Chem. Soc. 127, 2264 (2005).
AMANDA J. HAES is a National Research Postdoctoral Researcher in the chemistry division at the U.S. Naval Research Laboratory, Chemistry Division Code 6112, 4555 Overlook Ave, SW, Washington, DC 20375. W. PAIGE HALL is a graduate student in chemistry and RICHARD P. VAN DUYNE is a professor of chemistry at Northwestern University Chemistry Department, 2145 Sheridan Road, Evanston, IL 60208-3113; e-mail: [email protected].