Two laser-imaging techniques, one in clinical trials and the other in preclinical evaluation, may hold the long-sought key to diagnosis, treatment, and even prevention of Alzheimer’s disease and several related ailments, according to results that were scheduled for presentation at the annual meeting of the Optical Society of America (Tucson, AZ, Oct. 16-20).
Two and a half years ago, a paper published in The Lancet medical journal, with Lee Goldstein, a physician at Brigham & Women’s Hospital (Boston, MA) as lead author, indicated that β-amyloid peptides—which accumulate as plaque in the brain and are a major hallmark of Alzheimer’s disease—also deposit in the lens of the eye and correlate with the disease.1 The β-amyloid peptides form cataracts on the inner periphery of the lens, where they do not interfere with vision and tend to go unnoticed.
“This finding forces reconsideration of Alzheimer’s disease as a more global, perhaps systemic, problem, rather than one that is restricted to the brain,” Goldstein said. The presence of disease pathology in an optically accessible region of the body may solve a central problem, namely early detection and quantitative disease monitoring, he added. Currently, a definitive diagnosis of Alzheimer’s can only be made at autopsy. Diagnosing the disease in early stages relies on relatively difficult-to-interpret psychometric testing and brain scanning. The new developments raise the possibility of catching and perhaps treating the disease before the onset of irreversible brain damage.
“There are many hundreds of drugs in the pipeline for treating Alzheimer’s disease,” Goldstein said. “But the lack of a diagnostic test has posed a major impediment to getting those drugs into clinics, because there has been no way to evaluate effectiveness in preclinical animal testing, much less in human clinical trials.”
Combining QLS and FLS
Because no diagnostic method existed to look for β-amyloid peptides in the eye, Goldstein and colleagues set out to create one. They started with quasi-elastic light scattering (QLS), a sensitive technique originally developed by George Benedek and colleagues at the Massachusetts Institute of Technology (Cambridge, MA) for identifying light-scattering patterns within a fluid matrix, in this case for an aggregation of proteins in solution.
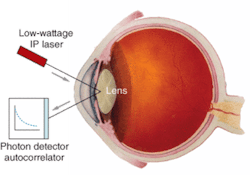
Goldstein’s research team harnessed the QLS method to examine the eyes of living patients, which required enabling the method to track numerous and diverse natural eye movements. The method uses a low-power IR Class I laser that pulses light into subregions of interest in the eye. Scattered light returns to the device for detection in photon counters and deconvolution in an autorcorrelator to ultimately indicate presence, location, quantity, and size of the microscopic aggregates within discrete regions of the lens (see figure). QLS enables identification of deposits well before they become hardened into visible cataracts, according to Goldstein.
While QLS is a sensitive tool for detecting microscopic aggregates in solution, it is not specific for amyloid peptides. So the researchers have coupled it with a less-sensitive but highly specific technique that Goldstein dubbed fluorescent ligand sensing (FLS). The technique relies on ligand molecules that are applied in eye drops, cross into the lens, and bind specifically to amyloids. Once bound, they fluoresce characteristically when excited by light at the proper wavelength. QLS is currently in investigational clinical trials, while FLS is in preclinical testing on laboratory animals. The technologies are complementary and a single instrument platform including both of them is being commercialized through a start-up company, Neuroptix (Amherst, MA).
In addition to enabling the diagnosis, study, and treatment of Alzheimer’s disease, the researchers also expect the new optical diagnostic methods to open up avenues of approach to other protein-aggregation diseases in humans and animals, such as age-related cataracts (the leading cause of blindness in the world, but not related to Alzheimer’s disease cataracts) and prion diseases, such as bovine spongiform encephalopathy (mad cow disease) in cattle and Creutzfeldt-Jakob disease in humans.
REFERENCE
1. L.E. Goldstein et al., The Lancet 361, 1258 (April 12, 2003).
About the Author
Hassaun A. Jones-Bey
Senior Editor and Freelance Writer
Hassaun A. Jones-Bey was a senior editor and then freelance writer for Laser Focus World.