Photonics Applied: Noninvasive Medical Diagnosis: Pursuing the tricorder: Noninvasive photonics-based health monitoring
EDIK U. RAFAILOV, EVGENY A. ZHEREBTSOV, SERGEI G. SOKOLOVSKI, VICTOR V. SIDOROV, and ILYA E. RAFAILOV
The matter of personal health is a concern shared by people around the world. A trip to the doctor is not something most people want to do—so what if they didn’t have to? The march of technological progress in the fields of optics and photonics has created a world wherein noninvasive health monitoring and diagnostics are no longer a figment of imagination in the minds of science fiction writers. Such devices and techniques are now being developed by real scientists and engineers.
Regular health checkups and blood tests are an effective way to ensure that possible complications do not go unnoticed—but unfortunately, not everyone has easy access to preventive care. More importantly, frequent visits to the doctor economically burden health systems already working at capacity. Consequently, not only do noninvasive technologies present an opportunity to catch deadly diseases at much earlier stages (leading to substantially better prognoses for the patients), but they also offer the possibility of rapidly monitoring many patients at the point of care, or even monitoring individuals at home without the need for doctor visits using handheld devices.1
The overwhelming potential behind handheld medical diagnostic and monitoring equipment was the impetus for the Qualcomm Tricorder XPRIZE (https://tricorder.xprize.org/prizes/tricorder) competition, which has so far awarded $4.7 million towards the development of a device that uses noninvasive technologies to diagnose a host of conditions, including sleep apnea, arterial fibrillation, pneumonia, urinary tract infection, and many others. Researchers and engineers are exploring the means to achieve such a monumental feat.
How do we get there?
Though a myriad of optoelectronic and chemical approaches is available, one of the primary and most effective noninvasive methods is through the application of photonics-based technologies. The interactions of light with numerous biological objects in an organic tissue forms the basis of all photonics-based technology used for biomedical applications.2
Because of the nature of light, however, the interaction with organic materials comes in three forms: photodestructive (where the thermal, hydrodynamic, and photochemical interactions of light cause destruction of tissue), photophysical/photochemical (where atoms and molecules within tissue are excited by light), and nonperturbing (where the light does not alter the tissue state). These interactions serve a surgical, therapeutic, or diagnostic purpose, respectively. But it is the latter that becomes so important when designing a noninvasive monitoring or diagnostic tool.
The techniques
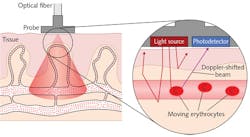
Laser Doppler flowmetry (LDF) has been an increasingly popular method for hemodynamics measurement since its commercialization in the early 1980s.3 This approach avoids the use of radioactive makers by analyzing the scattering of low-power, near-infrared (near-IR) radiation directed into organic tissue by endogenous molecules. A Doppler frequency shift is observed as the light interacts with the fast-moving erythrocytes (see Fig. 1). This shift is ultimately registered by photodetectors, enabling continuous and real-time measurement of tissue blood perfusion.
Though the LFD technique suffers from motion-related signal distortion and a reliance on arbitrary units, it has still seen extensive use in many areas, including physiological and cancer research.4, 5
Tissue oximetry (TO)—a technique most commonly used within research environments—is now being directed towards the noninvasive assessment of bio-tissues and organisms. The principle behind TO is dependent on the comparison of the oxygenated and deoxygenated fractions of hemoglobin within the microvasculature in the measured area of biological tissue.6 These fractions have unique characteristics of absorption and can be detected by different wavelengths of visible and near-IR light. This ultimately allows for the determination of oxygen transport and utilization as well as oxygen consumption within a studied living system.
Fluorescence spectroscopy is a technique whereby tissue samples are illuminated with specific wavelengths of light, causing fluorescence. The absorption of this light by the endogenous fluorophore molecules leads to their excitation, resulting in energy excitation and ultimately re-emittance of light at a longer wavelength.7 This longer-wavelength light is then monitored through surface-level spectrometers or spectrographs, producing a real-time, continuous fluorescence spectrum.
The fluorescence spectroscopy technique, however, faces challenges in overcoming the scattering events of the re-emitted light, especially in the presence of molecules such as hemoglobin that are known to be strong absorbers. Despite this, the relative content of many endogenous molecules—including metabolic cofactors like the reduced nicotinamide adenine dinucleotide (NADH) and oxidized flavin adenine dinucleotide (FAD), structural proteins like collagen and elastin, as well as others such as porphyrins—can be observed and monitored. Such monitoring can lead to not only raw information about the biomarkers, but from further analysis can be used to obtain a relative metabolic rate value.
Considering that NADH and FAD have different peak fluorescence and the process of metabolism involves the net gain of NADH and net consumption of FAD, the two biomarkers are functionally inverse. This means a ratio can be used to provide a numeric value describing the metabolism of an organic tissue and by following the fluctuations in said ratio, a general indication of increases and decreases in metabolic activity can be monitored.
It is precisely this level of information that has allowed fluorescence spectroscopy to be useful in detection of multiple diseases and conditions from atherosclerosis of the aorta and coronary arteries to multiple forms of cancer, including urinary bladder, breast, and lung.
Pursuing the tricorder
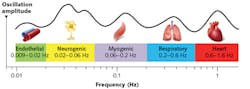
While the techniques described here are far from the only ones available for noninvasive assessment of organic tissues, they form a backbone to some of the latest technological developments in the noninvasive diagnostics field—for example, the study of vasomotion (see Fig. 2).Through the combined application of such technologies, even more informative data not available otherwise may be elucidated. These are also major areas of research in the UK by Aston University and its industrial partners. Individual application of such techniques as well as combined simultaneous use is one of the primary directions of research into noninvasive diagnostics at our institutions.
Furthermore, a new prototype is under development, integrating additional channels into the existing wearable system. Fluorescence spectroscopy presents itself as the best complementary technique, allowing for the expansion of biological tissue monitoring to include information about metabolism and other potentially key disease biomarkers.
Through the application of photonics techniques and packaging miniaturization, everyday health monitoring is possible without the need for a doctor. Even more exciting is the potential integration with the Internet of Things (IoT). Noninvasive mobile technology opens the door to the constant tracking of one’s health with their associated needs in the form of medications and/or fitness regimes.
REFERENCES
1. See https://bit.ly/1CeE0Vx.
2. K. S. Litvinova et al, Prog. Quant. Electron., 56, 1–14 (2017).
3. V. Rajan et al., Lasers Med. Sci., 24, 2, 269–283 (2009).
4. A.V. Dunaev et al., Physiol. Meas., 35, 4, 607–621 (2014).
5. D. M. Hemingway et al., Br. J. Cancer, 66, 958–960 (1992).
6. T. W. L. Scheeren, J. Clin. Monit. Comput., 26, 4, 279–287 (2012).
7. N. Ramanujam, “Fluorescence spectroscopy in vivo,” Encyclopedia of Analytical Chemistry, 20–56 (2000).
8. E. Zherebtsov et al., “Novel wearable VCSEL-based blood perfusion sensor,” International Conference on Laser Optics, St. Petersburg, Russia (Jun. 4–8, 2018).
Edik U. Rafailov is a professor, Evgeny A. Zherebtsov is Marie Curie Fellow, and Sergei G. Sokolovski is senior research fellow in the Optoelectronics and Biomedical Photonics Group, all at the Aston Institute of Photonics Technologies at Aston University, Birmingham, England, while Victor V. Sidorov is CEO at SPE Lazma, Moscow, Russia, and Ilya E. Rafailov is director at Aston Medical Technology, Birmingham, England; e-mail: [email protected]; www.aston.ac.uk.