An interdisciplinary team from Purdue University has shown that a combination of laser technology and nanotechnology that it originally developed for medical imaging also has therapeutic potential. The method causes tumor cells in laboratory cultures to self-destruct.
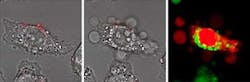
The procedure relies on tiny particles of gold, known as nanorods, and near-infrared (IR) light, which easily penetrates tissue. Researchers first use a pulsed IR laser to guide nanorods to the sites of tumor cells in vitro. They then switch the laser to continuous mode. When the nanorods absorb its light, they heat up (see figure). That increase in temperature sets off a sequence of reactions that kills the tumor cells.
Having demonstrated the combined technologies’ curative capabilities in cell cultures, the Purdue team has now started to apply the approach to animals. “We are working on how efficiently you can target a tumor impacted under the skin of a mouse,” says Ji-Xin Cheng, assistant professor of biomedical engineering at Purdue.
The team has also determined how the process kills tumor cells. “We have found that, rather than cooking the cells to death, the nanorods first punch holes in the membrane,” explains associate chemistry professor Alexander Wei. “Cell death is then chemically induced.”
Extensive animal testing and an understanding of the precise process involved in killing cancer cells are critical forerunners to any potential use of the method to treat cancer in human patients. Even if the technology proves suitable, it faces a long series of tests before it can make it into clinical practice. “Maybe it will be ready in ten years,” Cheng says. Nevertheless, the project has provided a boost to the concept that a combination of lasers and nanotech has medical value.
The Purdue team started the project by developing a method for locating gold nanorods in tissue. The 50 × 15 nm dimension of the chosen nanorods-about 1/200th the size of a red blood cell-has two advantages in terms of possible applications in human patients. It is small enough to stay in the bloodstream for significant lengths of time. And it has an aspect ratio of length to width that enables the nanorods to shine brightly when illuminated by near-IR light.
Tracking the nanorods
To guarantee high contrast and ultrabright images, the researchers adapted a two-photon fluorescence process in which a pair of photons hits a nanorod simultaneously. They injected nanorods into mice, and used a Mira 900 Ti:sapphire femtosecond pulse laser from Coherent (Santa Clara, CA) to produce images as the particles flowed through blood vessels in the ears of the mice. The result: images almost 60 times brighter than those produced by conventional fluorescent dyes.
Having developed a method of tracking nanorods, the team then looked at ways to locate them near to tumor cells. The membranes of tumor cells contain an unusually large number of receptor sites for folic acid, a form of vitamin B that many tumor cells crave. So the team attached folic acid to the nanorods. That helped to steer the nanoparticles to tumor membranes in cell cultures.
The two-photon process with the laser in pulsed mode was used to identify the positions of nanorods attached to the membranes of the tumor cells. Then the researchers switched the laser to continuous mode. In that state, the laser generates higher average power and heats the nanorods. As they heat up, the nanorods ionize the molecules around them. “This generates a plasma bubble that lasts for about a microsecond in a process of cavitation,” Wei explains. “Every cavitation is like a tiny bomb. Then, suddenly, you have a gaping hole where the nanorod was.”
In their initial experiments, the researchers waited for the nanorods to penetrate into the cells before heating them. They soon found, however, that they could kills the cells just as effectively, and at the expense of far less power, by exposing them to the continuous laser light while they were still on the membranes’ surfaces. “If you wait until the nanorods are inside the cells, you really have to pump up the laser power,” Cheng says. “So localizing the nanorods on the cell membrane strongly influences their ability to inflict cell damage.”
How does that damage occur? The researchers found that the nanorods cause the formation of blebs, similar to severe blisters, in the membranes. Rather than the heat alone, the blebs result from a chemical process. Heating the nanorods allows extra calcium to enter the cells. There, the team reports in Advanced Materials, it triggers enzymatic activity that loosens the cell’s internal infrastructure and creates the blebs in the membranes.1 That level of understanding will guide research teams as they try to adapt the process for use in the continuing battle against human cancer.
REFERENCE
1. Advanced Materials 19, 3136 (2007).
About the Author
Peter Gwynne
Freelance writer
Peter Gwynne is a freelance writer based in Massachusetts; e-mail: [email protected].