MEDICAL PHOTONICS: Light-activated agents overcome bacterial resistance
Scientists have proved that light-activated agents can render bacteria defenseless—even those that have become resistant to drugs. This is good news for the medical community, which increasingly runs into the problem of bacteria resistance, in which the microbes transform themselves in response to pharmaceutical treatments, effectively foiling attempts to defeat them.
Researchers at University College London (UCL; London, England), for instance, have proved the antibacterial properties of a human-safe dye, indocyanine green, in conjunction with near-infrared (NIR) laser light.1 Professor Michael Wilson, at the Division of Microbial Diseases within UCL’s Eastman Dental Institute, led the experiments that showed how activated indocyanine green is able to kill a wide range of bacteria, including staphylococcus aureus, streptococcus pyogenes, and pseudomonas aeruginosa.
“The mechanism of killing is nonspecific, with reactive oxygen species causing damage to many bacterial components, so resistance is unlikely to develop—even from repeated use,” says Wilson. The light activates the agents to produce cytotoxic species that can kill bacterial cells by inducing loss of membrane integrity or lipid peroxidation, by inactivating essential enzymes, or by exerting mutagenic effects due to DNA modification.
The laser-powered treatment promises to effectively treat infections that occur in wounds. “Infected wounds are responsible for significant morbidity and mortality, and an increase in the duration and the cost of hospital stay,” says Wilson, adding that the organisms causing these infections are increasingly resistant to conventional antibiotics. The laser the researchers used produces light that can penetrate even deep wounds. It emits high- or low-intensity 808 nm light, which is known to be capable of heating tissue. However, Wilson notes, “Substantial killing of all of the bacteria tested was achieved without causing any temperature rise.”
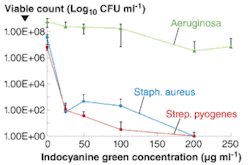
In the researchers’ studies, the bactericidal effect depended on both the concentration of indocyanine green and the light dose (see figure). Indocyanine green photosensitization using both high- and low-intensity (1.37 W cm—2 and 0.048 W cm—2, respectively) NIR laser light was able to achieve reductions of 5.6 log10 (greater than 99.99%) and 6.8 log10 (also greater than 99.99%) in the viable counts of staphylococcus aureus and streptococcus pyogenes (using starting concentrations of 106 to 107 CFU ml—1; a “CFU” is a “colony-forming unit,” a microbiological measure of the quantity of viable cells). Cell death rates of 99.99% were obtained for pseudomonas aeruginosa (initial concentration 108 to 109 CFU ml—1) photosensitized by the high-intensity light (1.37 W cm—2); while a kill of 80% was achieved using low-intensity irradiation (0.07 W cm—2). The effects of L-tryptophan (a singlet oxygen scavenger) and deuterium oxide (an enhancer of the life span of singlet oxygen) on the survival of staphylococcus aureus were also studied. L-tryptophan reduced the proportion of staphylococcus aureus killed; whereas deuterium oxide increased the proportion killed, suggesting that singlet oxygen was involved in the killing of the bacteria.
REFERENCE
- G.S. Omar, et al., BMC Microbiology, doi:10.1186/1471-2180-8-111 (July 1, 2008).
About the Author

Barbara Gefvert
Editor-in-Chief, BioOptics World (2008-2020)
Barbara G. Gefvert has been a science and technology editor and writer since 1987, and served as editor in chief on multiple publications, including Sensors magazine for nearly a decade.