Spectral measurements reveal atomic structure in fermium
Originally discovered half a century ago in debris of the first hydrogen-bomb explosion, fermium has 18 known isotopes, of which fermium 257, the most stable, has a half-life of only 100 days. It took almost two years for researchers at the Oak Ridge National Laboratory (Oak Ridge, TN) nuclear reactor to create nanogram amounts of fermium 255 with a half-life of 20.1 hours from which 1,718 ng were shipped to the University of Mainz (Mainz, Germany), where resonance ionization spectroscopy with an excimer-dye-laser combination along with mass analysis enabled observation for the first time in fermium of two calculated atomic levels above the ground state.1
Working with a sample of only 2.7 × 1010 atoms, the researchers had to initially narrow the search window using relativistic multiconfiguration Dirac-Fock calculations to predict appropriate levels at which to perform their two-step resonance ionization spectroscopy.
A tunable dye laser with an 8-GHz spectral linewidth enabled them to scan across a wavenumber range between 24,703 and 25,990 cm-1 containing two predicted atomic levels, during nonresident ionization with 15-ns, 50-mJ pulses from a 351/353-nm-emitting excimer pump laser operating at a 200-Hz pulse-repetition rate.
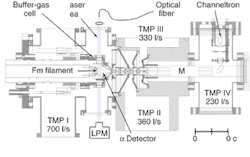
Under laser illumination, the fermium atoms could only be stored in the argon buffer gas for about 40 ms; in the course of an eight-hour scan, the researchers illuminated about one million atoms per second. After two-step laser ionization, a combination of an electric field and a gas flow transported the fermium ions into a quadrupole mass spectrometer for mass analysis and identification (see figure). Resonances were observed in the vicinity of two of the predicted energy states at wave numbers of 25,099.8 and 25,111.8 cm-1, but observing the predicted three higher energy states would have required a larger sample of fermium, according to the researchers.
Furthermore, the drift time of the ions through the buffer gas was determined. "In principle, a measurement of the drift time of the ions in the optical cell enables a determination of the ionic mobility and may open up avenues in studies of relativistic effects on ionic radii and the bond lengths of simple molecular ions," they wrote.1
REFERENCE
- M. Sewtz et al., Phys. Rev. Lett. 90(16) 163002-1 (April 25, 2003).