PHOTOVOLTAICS: Vibrational spectroscopy guides organic-solar-cell material design
Researchers at Pennsylvania State University (Penn State; University Park, PA) are using infrared vibrational spectroscopy to guide a material-design process that they hope will eventually lead to a new generation of organic photovoltaic (OPV) cells.
Organic photovoltaic materials have drawn interest for their potential to provide solar power that is less expense than traditional inorganic solar cell materials. Primary issues to be addressed, however, include efficiency and operational lifetime. The benchmark for useful solar-cell efficiency is about 10%; OPV materials currently offer only 3% to 4%—when they are brand new. Degradation upon prolonged exposure to sunlight occurs much faster than in crystalline solar cells.
Materials-design research to address these concerns has traditionally consisted of placing prospective OPV materials in a device, measuring their properties, and then repeating the process with other materials, said John Asbury, who leads the research team in the chemistry department at Penn State. “This is a blind multidimensional search process,” said Asbury. “It doesn’t take molecules into account, despite the fact that the issues of concern relate to molecular-level processes.”
The efficiency of an OPV solar cell is determined by the mobility of charge carriers and how efficiently they can be separated in the photoactive layer, a bulk heterojunction that provides the necessary charge separation in organic materials. Electron and hole pairs (excitons) need to find an interface where donor molecules can transfer electrons to acceptor molecules. So a high density of interfaces is required for efficient generation of electricity. “But charges also tend to get stuck in interfaces,” Asbury said. “So we need to understand what is happening at the interface.”
Exploring the structure
The traditional method of probing OPV interfaces, atomic-force microscopy (AFM), takes a high-resolution picture, but provides no molecular information and no information about structural dynamics or the movement of charge carriers, he said. Ultrafast two-dimensional infrared (2-D IR) vibrational spectroscopy, however, enables exploration of the structure and dynamics of condensed-phase systems through molecular vibrations.
So the Penn State researchers are supplementing their AFM studies of OPV bulk-heterojunction thin films with ultrafast 2-D IR spectroscopy, to essentially provide functional imaging.1 Their light source is an ultrafast Ti:sapphire laser pumping an optical parametric amplifier to produce 6 µJ, 150 fs mid-IR pulses at a 1 kHz repetition rate. The pulses were split into pump and probe beams, and phase-resolved 2-D IR experiments were conducted in the frequency domain.
The researchers are able to observe the three processes that are central to photovoltaic action-charge separation, charge transport, and charge recombination-through the vibrational modes of the molecules. The vibrational frequencies of molecules are very sensitive to the presence of charge carriers. By observing these processes they can diagnose the shortcomings of trial OPV materials and learn how to make better materials.
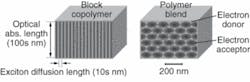
“The design problem involves learning how to separate the exciton diffusion length from the optical diffusion length,” Asbury said. “It presents unique challenges. The interface needs to be thick for optical absorption but thin for exciton diffusion.” To achieve this, they are developing a “spaghetti” structure composed of rod-shaped donor material surrounded by acceptor material. The rods are stacked parallel to each other to create a film whose thickness is comparable to the length of the rods. The excitons readily cross the short interface between donor and acceptor materials, while photons can be absorbed anywhere along the rods that provide the needed length for efficient optical absorption (see figure). The electrons can then race down the length of the rods to efficiently reach the electrodes.
Asbury’s team is currently collaborating to implement the spaghetti design with Qing Wang in the materials science and engineering department at Penn State. The focus is on developing ways to control geometry of electron acceptor and donor materials. The Wang group covalently bonds acceptor and donor polymers to each other in a block polymer. The researchers make one material hydrophobic and the other hydrophilic, so that they will phase-separate when cast together. “This enables us to control the length over which excitons must diffuse,” Asbury said.
The ultrafast pump-and-probe technique also enables direct examination of photochemical transformations associated with degradation in OPV materials. A femtosecond pump pulse stimulates the material to transfer electrons, and a probe pulse looks for new chemical species that can result on the nanosecond time scale and degrade efficiency, by setting up trap states for electrons and holes.
REFERENCE
1. L.W. Barbour et al., J. Phys. Chem. B 110(48) 24281 (2006).
About the Author
Hassaun A. Jones-Bey
Senior Editor and Freelance Writer
Hassaun A. Jones-Bey was a senior editor and then freelance writer for Laser Focus World.