UV SOURCES: Alexandrite lasers span the ultraviolet
DONALD F. HELLER
While mid-power excimer lasers have helped create major markets for several deep-ultraviolet (UV) applications, and low-power near-UV diode lasers are speeding advances in information storage and biotechnology, almost all commercial applications are taking place in substantially less than 1% of the UV spectrum, primarily near its extrema. In the middle lies vast potential largely associated with the identification, characterization, and manipulation of atomic and molecular electronic structure. Exploiting this region requires a new degree of knowledge and sophistication, and its exploration requires the use of more versatile laser sources.
Applications in basic physics, remote sensing, molecular detection, combustion research, and optical metrology have benefited from the recent evolution of tunable and fixed-wavelength UV sources based on alexandrite laser technology. The last decade has seen the realization of reliable UV laser sources that can sit on a narrow spectral feature or scan any required spectral region with nearly any desired wavelength, bandwidth, and temporal and spatial pulse characteristics.
Why UV?
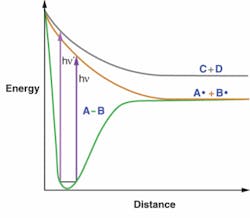
What sets the UV spectral region apart is the subtlety (and complexity) of the light-matter interactions indigenous to the realm. Here, where wavelengths range from 400 nm to somewhat below 200 nm, almost all but the smallest atoms and molecules absorb light, inducing changes-even if only very transiently-in their electronic structure. The associated increase in internal energy is usually sufficient to make, break, or rearrange chemical bonds (see Fig. 1) and to excite ionize (but rarely ionize) atoms. These processes are the very essence of chemistry.
At shorter wavelengths (in the vacuum-UV, extreme-UV, x-ray, or gamma-ray spectral regions) photons impart so much energy into their spectral “target” that electrons are typically ripped from atoms and molecules are fragmented into radicals and ions, usually having high degrees of internal energy. And because almost all solids absorb in these shorter-wavelength regions (often creating localized color centers or physical damage), the vast infrastructure of transmissive optics is of no avail.
Across the spectrum, opportunities for refined control of chemical (material) processes are tantalizing, but rare; the UV seems to hold the most promise. In lower-lying spectral regions, photon absorption produces internal atomic and molecular excitation, but much less chemistry. In the visible, where excitation is typically into low-lying electronic states, only the weakest molecular bonds can be broken. In the infrared (IR) region and below, absorption leads to vibrational or rotational excitation that is rapidly degraded to heat. While heating is usually insufficient to break most bonds in isolated molecules, thermal bond breaking can occur if the light intensity is high enough to induce multiphoton absorption or if multiple-photon absorption increases local temperature sufficiently for thermochemistry to occur.
Unlike thermochemistry-a typically nonspecific process that generates identical product distributions independent of how the energy was deposited into the reactants-the photochemistry that occurs in the UV (and to some degree in the visible and near-IR spectral regions) is highly specific in its deposition, frequently leads to nonthermal products, and can lead to differing products controlled by the frequency and the temporal coherence of the light (see Fig. 2).1 Molecules in gases, and even in condensed phases, display unique (although often overlapping) absorption features and sometimes emit or scatter light with their own unique spectral signatures.
This rich detail has led to a host of sensitive methods for detection of molecular species at low concentrations including cavity-ringdown and Raman spectroscopy, laser-induced fluorescence, cold-vapor atomic fluorescence spectroscopy, matrix-assisted laser desorption/ionization (MALDI), laser-induced-breakdown spectroscopy (LIBS), and various light-detection and ranging (lidar) techniques for remote sensing. Many of these technologies are now standards in analytical chemistry, bioassay, and forensic laboratories, or are used in new procedures for pollutant analysis or atmospheric monitoring.2, 3, 4
Although UV and other laser sources are commonly used for certain materials-processing applications, laser-based methods have been slow to gain traction in commercial photochemical production and processing. Despite their potential advantage for product-selective or even bond-selective chemistry, these sources have a relatively high cost per photon.5 Laser use today is restricted to photochemical processes in which product values are high, such as in UV photolithography and production of single-walled nanotubes (see www.laserfocusworld.com/articles/231725).6 The broader adoption of UV lasers awaits the development of cheaper, more amenable sources.
Better UV sources
Until relatively recently, the lack of good or even reasonable UV laser sources was a major barrier to scientific discovery and applications development. Excimer lasers, nitrogen lasers, ruby, and Nd:YAG laser harmonics are restricted to very specific wavelengths and-for excimer and nitrogen lasers-to sources with typically poor beam quality (generally indicative of very-high-gain, high-pulse-energy laser devices). Dye lasers and optical parametric oscillators and their associated harmonics provide tunable sources, but their use is rarely routine, even in laboratory settings.
In the 1980s, the first broadly tunable solid-state lasers able to operate routinely at ambient temperatures were introduced: the vibronic lasers alexandrite and Ti:sapphire. Although many other vibronic laser materials were discovered, none has proved capable of widespread commercialization. The Ti:sapphire laser has helped open doors into the ultrafast world of chemical dynamics. According to sources at Northrop Grumman/Synoptics, alexandrite is now the second most commonly used solid-state laser material after Nd:YAG (laser diodes and fiber lasers, while solid-state devices, are usually not included in this classification).
The alexandrite laser
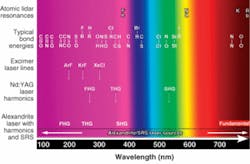
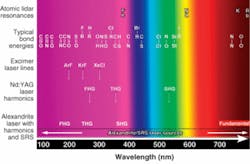
With a fundamental output from 701 to 860 nm, the alexandrite laser is principally used near the center of its gain region between roughly 720 and 800 nm. Its principal harmonics span the 360 to 400, 240 to 270, and 190 to 200 nm wavelength ranges; the fourth-harmonic range is limited by the lack of suitably transparent harmonic-generation crystals. Like Nd:YAG lasers, most alexandrite lasers are optically pumped by flashlamps, but can also be pumped directly by diode lasers. While the diode-pumped lasers have higher efficiencies, the relatively high cost of the pump diodes makes flashlamp pumping more economical for most commercial applications.
Harmonic-generation efficiencies for alexandrite lasers are comparable to Nd:YAG lasers. The development and refinement of wavelength conversion by stimulated Raman scattering (SRS) has made the alexandrite laser useful for UV (and other) regions outside of its harmonics. Attempts at commercializing early devices based on SRS were made in the late 1970s and early 1980s, but a number of factors were not understood at that time. Consequently, these devices exhibited poor conversion efficiencies, beam quality, and stability, and were eventually abandoned.
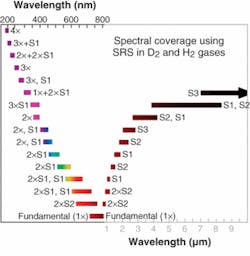
Subsequently, the physics of SRS became better understood and methods were developed to mitigate the early problems. A new class of Raman conversion devices, such as the Light Age 101PAL/RC, emerged and were commercialized. While these converter products were initially used as an enhancement for alexandrite laser systems, they are now widely used with most other pulsed laser sources as supplements and as replacements for less-robust alternatives. In conjunction with tunable alexandrite laser pump sources, these wavelength converters provide substantial output spanning the entire UV spectrum and may be the only viable sources for obtaining universal IR coverage down to at least 18 µm (see Fig. 3). Coverage of the entire visible spectrum is incidental.
Other developments have made the alexandrite laser, and its wavelength-converted output, unique and enabling for applications such as high-resolution spectroscopy and remote sensing. Foremost among these developments was the development of diode-laser injection seeding (DLIS). In this technique, the single-longitudinal-mode output of the laser is locked by active cavity-length stabilization (ACLS) to the wavelength of a low-power (few milliwatt) continuous-wave diode-laser source. The wavelength and frequency stability of the output is set by the diode laser; scanning the diode-laser frequency or locking it to some particular frequency reference or absorption feature similarly scans or locks the alexandrite laser wavelength, and consequently the harmonics and Raman-derived outputs. The DLIS technique also permits the bandwidth of the laser, as well as its frequency, to be controlled or tuned. At fixed bandwidth the output can be scanned continuously over large ranges with megahertz resolution, even when converted into the UV or IR (see Fig. 4).
Using DLIS in commercial alexandrite laser systems has provided Doppler-free UV molecular spectra at resolutions down to 8 MHz. These systems operate with high stability over extended periods of time. They have been used over several years at the South Pole in atmospheric lidar to measure mesospheric pressure, temperature, and species concentrations; in effect providing atomic spectroscopy on regions 40 to 80 km away.7 Similar systems have permitted high-resolution measurements and tests of fundamental physics, the writing of sub-30 nm structures and measurements of high-precision optics for advanced photolithography by 193 nm interferometry, and advanced methods for detection and monitoring of mercury and other environmentally important species.8, 9, 10
REFERENCES
1. S.A. Rice and M. Zhao, “Optical Control of Molecular Dynamics,” Wiley Interscience, New York, NY (2000).
2. K. Tanaka et al., Rapid Comm. in Mass Spectrometry 2, 151 (1988).
3. P. Petersson et al., to be published in Applied Optics (2007).
4. M.R Hammond et al., Proc. SPIE 4484, 103 (2001).
5. J.D. Baldeschwieler and G.C. Pimentel, J. Chem. Phys. 33, 1008 (1960); R.G. Bray and M.J. Berry, J. Chem. Phys. 71, 4909 (1979); R.B Metz et al., J. Chem. Phys. 99, 1744 (1993); S.A. Kandel and R.N. Zare, J. Chem. Phys. 109, 9719 (1998); A.H. Zewail, Nobel Prize Lecture (1999).
6. K.E.H. Gilbert et al., Appl. Phys. Lett. 88, 143122 (2006); A. Anctick et al., APS March Meeting, Abstract: R1.00046 (2007).
7. X. Chu et al., Appl. Optics 41, 4400 (2002).
8. V. Meyer et al., Phys. Rev. Lett. 84(6) 1136 (2000); J. Hoffner and J.S. Friedman, Atmos. Chem. Phys. Discuss. 4, 399 (2004).
9. E.S. Fry et al., Physica Scripta T76, 47 (1998).
10. U.S. Office of Water Environmental Protection 4303, EPA-821-R-02-019 “Mercury in Water by Oxidation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry” Method 1631, Revision E (August 2002).
DONALD F. HELLER is CEO of Light Age, 500 Apgar Drive, Somerset, NJ 08873; e-mail: [email protected]; www.lightage.com.