BIOMEDICAL IMAGING: OCT images the developing heart in vivo
MICHAEL W. JENKINS and ANDREW M. ROLLINS
Congenital heart disease affects 36,000 babies born in the United States annually. The causes of these abnormalities are largely unknown because of a lack of adequate tools to assess the genesis of these cardiac anomalies. Avian (chicken and quail) and murine (mouse) hearts develop similarly to humans and have become the most popular animal models in heart-development research. The gestation period for both avian and murine embryos is 21 days. At the onset of beating, the heart exists as a straight tube that moves blood by peristalsis, much as the gut moves food. Over the next couple of days, the heart rapidly grows and transforms into a four-chambered pulsatile pump through processes known as looping and septation. It is in this dramatic time period where many abnormalities can begin to manifest.
Because of the small size of the embryonic heart (less than 2 mm across), standard modalities for imaging internal structures, such as magnetic-resonance imaging and ultrasound, do not have adequate resolution, especially when investigating early development. This has left researchers to rely upon bioassays, video microscopy, and modeling to answer their most poignant questions. Clearly, an in vivo imaging tool with high spatiotemporal resolution and an appropriate field of view for the looping stages (millimeters) would be important for illuminating how the heart develops.
OCT
Optical-coherence tomography (OCT) measures coherently back-scattered light to generate cross-sectional images of biological tissue.1 With the use of low-coherence and near-infrared light, resolutions of 2 to 15 µm to a depth of 1 to 2 mm in cardiac tissue can be achieved, making OCT ideal for studying the developing heart. Optical-coherence tomography has been used to image the embryonic heart in several animal models, including the avian embryo and murine embryo.2-5
Because OCT is capable of very high imaging rates, it offers the ability to examine tissue morphology dynamically, as well as statically. We are developing OCT technology for a high-throughput phenotyping (classifying the outward physical manifestation and behavior of an organism) system and methods capable of four-dimensional (4-D) longitudinal imaging of early embryonic hearts. These technologies have the potential to expand our understanding of the genetic and epigenetic mechanisms that drive normal and abnormal heart development.
Phenotyping
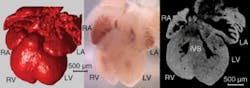
Manipulation of the genome has allowed researchers to identify genetic mechanisms of disease and development. The mouse is currently a widely used animal model for genetic manipulations because it is a mammal with a four-chambered heart, has a short gestation period, and its genome has been completely mapped. Optical-coherence tomography has been used to characterize morphological phenotype in developing hearts (see Fig. 1).6 With OCT one can phenotype the heart without destroying the tissue, thus allowing further studies. Being able to quickly phenotype a large population of mice nondestructively would advance genetic studies at a faster rate. Pinpointing the time when the defect can be first detected is crucial to understanding the etiology of heart defects.
We used OCT to characterize the morphological phenotype of murine hearts discerning hexamethylene bisacetamide-inducible protein 1 (HEXIM1) mutants from their wild-type littermates. At day 12.5 and 13.5 murine embryos were excised from the mother, the hearts were removed and three-dimensional (3-D) OCT image sets were obtained from each heart in the litter. Next, the morphological borders were segmented to obtain cavity volumes and wall thicknesses. The mutant hearts exhibited increased ventricular chamber volume and decreased compact myocardium wall thickness when compared with their wild-type littermates. Histological analysis on similar hearts reveals comparable results. This study demonstrated that OCT imaging can assess the 3-D morphology of the embryonic heart much more rapidly than by histology, and without destroying the sample.
4-D imaging
Although the speed of OCT imaging systems has increased more than 20-fold in the last several years, the systems are still not fast enough to capture the dynamics of the beating heart in three dimensions during the rapidly contracting phase of the heartbeat (systole). Simultaneous imaging of very early embryonic heart structure and function has technical limitations in terms of spatial and temporal resolution. Members of our research team approached the problem by developing cardiac-gating methods to build up 4-D (x, y, z, and time t) image sets over many heartbeats and we have constructed an OCT system featuring a Fourier-domain modelocked laser (FDML). The laser allows us to image at extremely high speeds (100 kHz line rate) and enables OCT systems to assess structure and function simultaneously.7, 8, 9 Visualizing the heart in four dimensions enables researchers to quickly pinpoint areas of interest and see how these areas relate to their environment.
We first demonstrated prospectively gated OCT imaging of excised chicken and mouse hearts by electrically pacing the hearts and using the pacing signal to trigger the data acquisition. Advanced image-processing techniques were used to visualize the hearts in four dimensions and measure physiological parameters such as cardiac volume, ejection fraction, and wall thickness. While effective, this technique was not appropriate for imaging the heart in a living embryo.
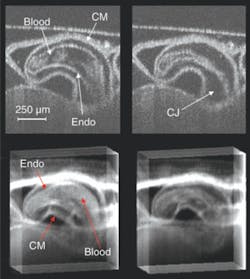
Medical cardiac-gated imaging modalities derive a gating trigger from the electrocardiogram (EKG) of the patient. Unfortunately, the electrical signal from a preseptated embryonic heart is minute and to record it electrodes would have to be placed virtually in contact with the heart, which is invasive from the perspective of the embryo. The precise placement of the electrodes is extremely challenging and it is not clear that adequate signals can be obtained from early embryos. To circumvent this problem we derived a gating signal using laser Doppler velocimetry (LDV) to noninvasively extract a flow signal from one of the outer vitelline vessels. As a demonstration, we recorded a 4-D image set of an in vivo beating quail heart (day two of development) consisting of 864,000 axial scans over multiple heartbeats. The data set represents full 3-D volumes at eight time points through the heart cycle (see Fig. 2).
The OCT sum-voxel projection images (or slices) of the heart in diastole and systole clearly resolve important anatomical features such as the compact myocardium and the endocardium, and demonstrate the image quality achievable with this technique. Sum-voxel projection allows us to easily visualize and interpret a 3-D image, revealing the outer and inner surfaces of the heart and how the heart is positioned within the surrounding tissue. The 3-D structure of the heart becomes further evident by projecting along various angles to visualize the volume while it rotates.
The recently developed FDML lasers have enabled unprecedented OCT imaging speeds.10 The high scan rate of these systems enables the acquisition of high-temporal-resolution 2-D image sets (195 frames per second) and 3-D datasets (10 volumes per second). The development of our FDML laser OCT system enabled, for the first time, an in vivo 4-D image of a preseptated embryonic avian heart without the aid of gating. This was also the first time the embryonic heart could be viewed in cross section during looping with extremely high temporal resolution, enabling the observation of morphological dynamics of the beating heart during systole (less than 50 ms in duration). Incorporating cardiac gating into the high-speed system will enable recording of 4-D image sets at 200 volumes per second, which will allow us to visualize and study systolic dynamics in three dimensions.
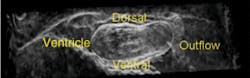
Images of a 2-day-old quail heart taken from a four-dimensional image set (with borders enhanced prior to volume rendering) demonstrate a visualization method that produces clearly interpretable results without resorting to time-intensive manual segmentation (see Fig. 3). This approach would be suitable for high-throughput and rapid preliminary phenotyping of internal and external embryonic structures, revealing the cardiac interaction with surrounding tissue.
Clearly, OCT imaging is well suited to examining and phenotyping the living, beating, developing heart. The ability to examine the embryonic heart in vivo over time will enable new experiments previously not possible and has the potential to answer important questions regarding normal development of the heart, as well as aid in discovering the mechanisms that lead to heart defects.
REFERENCES
1. D. Huang et al., Science 254, 1178 (1991).
2. S.A. Boppart et al., Proc. Natl. Acad. Sci. USA 94, 4256 (1997).
3. A. Mariampillai et al., Optics Express 15, 1627 (2007).
4. T.M. Yelbuz et al., Circulation 106 (22) 2771 (November 2002).
5. W. Luo et al., J. Biomed. Optics 11, 021014 (2006).
6. M.W. Jenkins et al., Applied Optics 46, 1776 (2007).
7. M.W. Jenkins et al., Optics Express 14, 736 (2006).
8. M.W. Jenkins et al., Optics Express 15, 6251 (2007).
9. M.W. Jenkins et al., J. Biomed. Optics 12 (3) 030505 (2007).
10. R. Huber, D.C. Adler, and J.G. Fujimoto, Optics Lett. 31, 2975 (2006).
Michael W. Jenkins is graduate research assistant and Andrew M. Rollins is Warren E. Rupp Associate Professor in the Departments of Biomedical Engineering and Medicine at Case Western Reserve University, 10900 Euclid Ave., Cleveland, OH 44106; e-mail: [email protected]; www.case.edu.