From microscopy to surgery, lasers pervade biomedicine
A biologist examining a cell under a microscope, a surgeon correcting the eyes of a nearsighted patient, a doctor finding a tumor, a biochemist tracing a reaction in a cell—these procedures are all likely to use lasers routinely. Lasers have become ubiquitous in biological research and in medical practice, with dozens of major applications, most of them using standardized commercial equipment. With the commercialization and rapid progress of ultrafast lasers, new applications using such lasers are rapidly moving into the biomedical marketplace.
A three-dimensional view of cells
Of the many applications of lasers in biological research perhaps none has become more widespread than confocal microscopy. The microscope has, since Leuwenhook, been one of the prime tools of biology and since the first commercial models of laser scanning confocal microscopes were made 15 years ago, the new types have increasingly emerged as a standard piece of equipment in biology laboratories.
Confocal laser-scanning microscopes (CLSM) allow sharp imaging of three-dimensional objects, in contrast to conventional microscopes that only allow imaging in a single focal plane. In a CLSM, light from a laser is focused by a lens and passes through a pinhole aperture before being focused on the specimen. Light from the specimen, either reflected or fluorescent, then passes through the same focusing lens, which acts as both objective and condenser, and is diverted by a beamsplitter to another pinhole aperture. The amount of light passing through the second pinhole is then converted to an electrical signal by a photomultiplier tube.
The confocal optical arrangement allows only light from the focal plane to pass through. Light above or below that plane is blocked by the pinhole apertures. This is in contrast to a conventional microscope that allows out-of-focus images from off-focal-plane layers to be seen.
To produce an image, the device is scanned over the entire specimen at a single height and then is shifted vertically in steps as small as 50 nm to repeat the scan and build up a three-dimensional image of many layers. A computer combines the many layer-images into a single three-dimensional image that can be viewed in many ways.
Typically confocal microscopes are based on krypton/argon ion lasers emitting at 488, 568, or 674 nm. Often a dye is added to a sample or a fluorescent signal is analyzed as output from the sample.
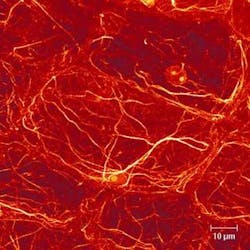
The advent of the confocal microscope has radically transformed the study of the structure of cells, with the actual three-dimensional relationship of objects in the cell becoming clear for the first time—revealed in often striking microphotographs (see Fig. 1).1
Optical tweezers pin cells
Not all cells that biologists study are part of tissues. Often they are free-floating in a nutrient bath. But an individual cell in a nutrient solution, such as a yeast cell, moves around continuously, buffeted by Brownian motion. To study it with a microscope or spectroscope, the cell must be pinned down without being damaged. For this purpose, laser optical tweezers are becoming increasingly common.
While the first experiments that showed bacteria and other small cells could be trapped by optical tweezers were performed in the late 1980s, commercialized versions are only now entering routine use in most biology labs. Such tweezers use the gentle pressure from photons in a laser beam to trap cells safely. Only tiny amounts of power (~2 mW) are required for the trapping, avoiding damage to the cells.
Recent work has greatly expanded the usefulness of optical trapping in cell studies by allowing the simultaneous use of Raman spectroscopy.2 Raman spectroscopy is extremely useful to biochemists, with a single spectrum revealing the presence and relative concentrations of dozens of chemicals. However, Raman spectroscopy requires much higher laser powers (20 to 100 mW) because the Raman scattering that couples photons with phonon vibrations is weak. Such a high level of radiation rapidly damages the cells.
Yong-qing Li and his colleagues at East Carolina University (Greenville, NC) solved this problem in a conceptually simple manner. For most of the time that a cell is trapped, the diode laser (operating at 785 nm) produces only the 2-mW output needed for immobilization. Only for short periods, two seconds at a time, does the laser switch to the higher 20-mW power needed to take the Raman spectrum. The exposure is too short to damage the cell and sufficient time is allowed before the next exposure, so damage is not cumulative.
Ultrafast surgery
The development and commercialization of femtosecond lasers has opened up new applications in biomedicine. Particularly in surgery, femtosecond pulses allow for far more precise cutting than did nanosecond lasers.3 Precise laser cutting is performed by photodisruption, which produces much less damage to surrounding tissues than photoablation, the vaporization of tissue. In photodisruption, a short highly focused laser pulse generates such a high electric field that atoms are torn apart, producing a plasma. The hot plasma expands rapidly, creating a bubble in the tissue. The whole process is over before any significant amount of heat has time to damage surrounding tissues.
The shorter the pulse, the smaller the amount of energy required for photodisruption and the smaller the size of the cavity produced, hence the finer the cut. While a 10-ns pulse produces a cavity 300 to 1200 µm across, a 60-ps pulse creates one 30 to 120 µm across and a 500-fs pulse creates one only 3 to 13 µm across. The fluence required drops from 185 J/cm2 for the nanosecond pulse to only 1.6 J/cm2 for the femtosecond pulse.
In one of the most common forms of laser therapy—corneal surgery to correct refractive problems like nearsightedness and astigmatism—a femtosecond laser is scanned across the area that needs to be cut at kilohertz pulse repetition rates. Since the cornea is transparent, the laser can cut underneath the surface at any depth, allowing the overlay tissue to be removed by suction. The femtosecond laser creates cuts that are far more precise and controlled than those made with longer-pulse lasers or with mechanical blades, thus improving results and reducing complications.
Seeing through tissue
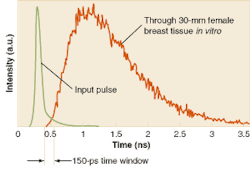
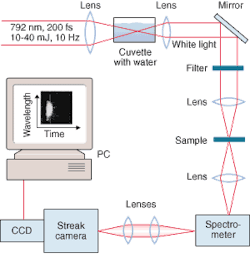
Ultrashort laser pulses have also opened up the whole field of optical tomography and other medical applications that involve scanning tissue optically. The basis for these techniques is the detection of photons that travel ballistically through tissue, without scattering. While these are a small fraction of all photons, because they travel in straight lines they arrive earlier at the detector than do the scattered photons. With sufficiently short pulses (around 30 ps) the ballistic photons can easily be separated from the slower scattered ones (see Fig. 2).The same concept is beginning to be applied to x-ray images of living subjects. While x-rays travel through tissue far more easily than light photons, there is still some scattering, which reduces contrast and resolution. Time gating of x-ray pulses can eliminate the scattered photons and produce sharper images. To generate x-ray pulses of a few picoseconds, a team at the Lund Institute of Technology (Lund, Sweden) uses a commercial terawatt laser emitting a pulse of 110 fs in length. The pulse, if focused on tantalum or other high-atomic-charge material, produces a range of x-ray energy around 60 keV. The studies showed that with time gating, contrast was enhanced and the total x-ray dose could be reduced.
While the x-ray pulse system is still in the laboratory, it is based on commercial lasers. Recent experience suggests that new laser techniques are moving from laboratory to commercial use in biomedicine at an accelerating pace, so the number of applications for ultrashort pulses is likely to expand greatly in the next decade.
REFERENCES
- E. Davies et al, Plant Sci. 160, 185 (February 2001).
- C. Xie et al, Opt. Lett. 27, 249 (February 2002).
- T. Juhasz et all, Opt.and Photon. News, 24, (January 2002).
- S. Svanberg, Meas. Sci. and Tech. 12(11), 1777 (November 2001).
Multilaser device offers diagnostic advance
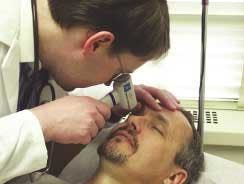
VitalCare Diagnostics, a collaboration between Sarnoff (Princeton, NJ) and Vectranetics (founded by a group of researchers from the University of Alabama; Huntsville), is working to commercialize a laser-based diagnostic tool that noninvasively measures oxygenation levels in the brain via the retina. Unlike other vital signs, which offer trailing indicators of patients in distress, an accurate measurement of brain oxygen use could provide a leading indicator of life-threatening physical distress within a patient before the situation becomes critical, sometimes even before the event occurs.
The handheld Eye Oximeter works by taking a spectroscopic measurement of blood oxygen saturation, passing multiple wavelengths of light through the veins and arteries of the retina separately. The system needs to determine four parameters: the oxygen saturation, the hemoglobin concentration, the vessel diameter, and a scattering coefficient. The device uses four wavelengths (488, 635, 670, and 830 nm). The 635-, 670-, and 830-nm sources are diode lasers, while the 488-nm source is an argon laser. However, the researchers plan to use an upconversion system with diode pumping to achieve the 488-nm wavelength in future versions of the instrument.While this sort of technique has previously been used for pulse oximetry, the retina's accessibility allows the oxygenation within arteries and veins to be measured separately. Additionally, the retina shares its circulation with the brain, yielding a more accurate measurement of brain oxygenation and metabolism.
In collaboration with Sarnoff, the researchers are now ramping up to begin human clinical trials. They believe a clinical prototype will be available in a year and a commercial device on the market in two years. Potential applications include trauma, diabetic retinopathy, early stroke-risk screening, glaucoma, and hypertension.—Kathy Kincade
Fly-through approach images tumors in real time
Using a combination of lasers, detectors, and holographic film, Purdue University (West Lafayette, IN) scientists have achieved the first visual fly-throughs of a living tumor, showing the inside of the tumor in real time.
Optical coherence imaging (OCI), a variant of optical coherence tomography (OCT), uses dynamic holographic film developed by the team to construct a coherence filter attached to the front of a standard video camera, passing only coherent, image-bearing light and rejecting scattered background, providing direct images without any computed reconstructions. In addition, because it uses short-coherence light, it is depth-gated, making it possible to "fly through" scattering tissue as a succession of cross sections.
Holographic coherence-domain imaging records full-frame depth-resolved images throughout living multicellular tumor spheroids in vitro, without computed tomography, allowing real-time video fly-through under interactive control of the operator. This imaging technique uses adaptive optics (a dynamic holographic film) to act as a coherence filter on a conventional video camera. The holographic camera ignores the intense scattered light that would overexpose ordinary film.
Led by David Nolte, a professor of physics at Purdue, the research team used a modelocked, 840-nm Ti:sapphire laser manufactured by Clark-MXR (Dexter, MI) in conjunction with the holographic film to take video images inside a cancerous rat tumor. They viewed an entire slice of the tumor at the same time without scanning and photographed it with a video camera running at 30 Hz. The researchers directed two crossing laser beams onto the film, creating holographic images.While most light detectors do not detect coherent light, the holographic film, which comprises alternating layers of gallium arsenide and aluminum gallium arsenide, is sensitive to coherent light. These semiconductors form a 200-layer film. Each layer is 8 nm thick, smaller than the wavelength of an electron in the material. Forcing electrons to move through such thin layers enhances the optical properties of the film and makes it more efficient at detecting coherent light. The holograms adjust to the changing light conditions and the changing information that is carried on the laser beams, creating a three-dimensional image.—Ilene Schneider
About the Author
Kathy Kincade
Contributing Editor
Kathy Kincade is the founding editor of BioOptics World and a veteran reporter on optical technologies for biomedicine. She also served as the editor-in-chief of DrBicuspid.com, a web portal for dental professionals.