Study of ice questions the origin of life
Based on Fourier-transform IR (FTIR) and laser-desorption laser-ionization mass-spectrometry studies of water ice at 10 to 15 K, Max Bernstein from NASA Ames Research Center (Moffet Field, CA) and other NASA researchers are finding evidence to support the theory that many biologically important chemicals on Earth, or at least their precursors, may have actually come from abiotic (not involving life) reactions in the interstellar medium.
Bernstein’s previously published research includes UV/ice reactions with various biologically interesting molecules including amino acids, polycyclic aromatic hydrocarbons (PAHs), and molecules that form into simple membrane-like structures found in organic extracts of certain meteorites.1, 2 His latest results show evidence that some important biochemical precursors for life formed abiotically via UV radiation of ice and PAHs.
Polycyclic aromatic hydrocarbons represent a significant (approximately 20%) contribution to cosmically available carbon.3 They are biologically interesting because their chemical structures are closely related to many biochemically important molecules-specifically co-enzymes for electron-transfer reactions, which are essential for biochemical metabolic functioning.
The experiment begins with simultaneous vapor deposition of water and PAHs onto a rotatable cesium iodide substrate in an evacuated chamber at 10‑K. The reaction chamber is coupled to an FTIR spectrometer. After deposition, the IR spectrum of the mixture is measured. The sample is then irradiated with UV light from a microwave-powered flowing hydrogen-discharge lamp that produces on the order of 2 × 1015 photons cm−2 s−1. The flux is nearly evenly distributed between the hydrogen Lyman alpha line and a roughly 20-nm-wide molecular transition centered at 160 nm. The flux is low enough that only single-photon processes are relevant. The photochemical changes in the PAHs are monitored using FTIR.
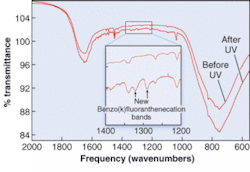
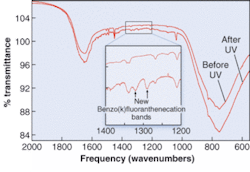
In one example, spectra are found for benzo[k]fluoranthene (see figure). “Not only is this the first time anyone has measured the IR spectrum of a PAH cation in H2O ice, but it allows us to predict that they are present in the interstellar medium. This prediction will be tested by the next generation of IR telescopes,” says Bernstein.
NASA missions exploring other planets will be looking for evidence of life, so Bernstein and his group are simulating conditions that would be present on other planets-in this case, the intense cold and radiation of Jupiter’s moon Europa. The goal of these experiments is to explain what is seen in meteorites, where interstellar-ice photochemistry is believed to be a key player.
They will be looking for evidence of life, but most likely won’t find actual organisms. They may, however, find chemical evidence of life-either currently thriving, or extinct organisms. Such “biomarkers” are the focus of Bernstein’s research.
“Chemical evidence of life would be the presence of biomarkers-compounds thought to be produced by biology, and their detection would ideally, be clear evidence of life,” says Bernstein. “Like finding an ancient stone ax head, these molecular remnants of past life would be proof that it had been there, and perhaps would tell us how long ago. But just as an amateur can confuse a stone shaped by natural forces with a real ax head, we are learning that extraterrestrial samples often contain compounds that look like those from life, but are not.”4
REFERENCES
1. M. P. Bernstein et al., Science 283, 1135 (1999).
2. J. P. Dworkin et al., Proc. Natl. Acad. Sci. USA 98, 815 (2001).
3. L. J. Allamandola et al., Astrophys. J. Suppl. Ser. 71,733 (1989).
4. M. Bernstein, Explorer, SETI Institute 1(1) 7, (2004).
lori howeis a freelance writer living in Mountain View, CA; e-mail: [email protected].