Hyperspectral imaging endoscope shows promise for in vivo cancer detection
Despite the abundance of research in optical methods for in vivo cancer detection, few commercial products are available to clinicians that provide both detection and treatment in the same instrument. This dual capability is a frontier being explored by scientists at Cedars-Sinai Medical Center (Los Angeles, CA) in cooperation with colleagues in the departments of pathology and bioengineering at the University of Pittsburgh (Pittsburgh, PA). The goal is to improve minimally invasive surgery by creating optical equipment that detects disease and delivers improved treatments at the site of the identified problem. The team’s current innovations in spectral optical imaging, in particular imaging elastic scattering spectroscopic (IESS) endoscopy, are being directed at a clinical trial focusing on lung cancer.
The new hyperspectral imaging endoscope (HSIE) developed by the team allows for noncontact rapid acquisition of polarized spectral images of tissue in the body.1 Building on work by previous researchers, the technique uses the Mie theory of scattering to estimate the size of particles in the tissue touched by the ends of an endoscopic probe. The scientists are improving on this point estimate by extending it to a noncontact full 2-D imaging modality enabled by the unique optical design of their HSIE probe. While systems do exist that acquire spectral imaging information in less than a minute, these are limited to point analysis rather than the 2-D imaging that would be required at the bedside to sample an entire tissue area of interest.
“Beautiful work was done by very smart people on the point/spectroscopic implementation, but the surgeons will not go with this,” said Daniel Farkas, project leader and director of the Minimally Invasive Surgical Technologies Institute at Cedars-Sinai. “We want to have the spectral imaging provide an ‘on-the-run’ objective alternative/supplement to pathology to enable better decisions for biopsy, treatment, and follow-up.”
According to theory, developed by Gustav Mie in 1908, it is possible to estimate the size of scattering particles-in this case, nuclei of the cellular monolayer of lung epithelial tissue-by shining light on the target tissue and analyzing the wavelength-dependent intensity (spectral oscillations) and polarization output from the sample under test. By adjusting the polarization, the tissue depth of the sampled area can be controlled.
Mostly off-the-shelf
With the exception of the specialized endoscope and the synchronization electronics, the HSIE system was built using off-the-shelf components. The 1.5-m-long imaging probe with 2-mm outside diameter, built in collaboration with Instrument Technology (Westfield, MA), incorporates a solid-core illumination fiber that delivers light from a monochromator in the range of 380 to 690 nm in 10-nm increments. A newer version uses custom acousto-optic tunable filters for wavelength control, extending into the near-IR. The probe then uses its two imaging fiber bundles with crossed polarizers to acquire the reflected spectral information (32 images) in a quarter of a second from tissue in an area approximately 8 mm in diameter.
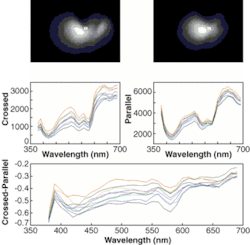
Using two imaging fibers, two orthogonally polarized images are placed side-by-side on the camera chip, each image containing approximately 5000 discrete pixels. An area of the field imaged by the standard endoscope can thus be spectrally imaged and diagnosed, using a mathematical model that views the tissue under test as a layer of single scattering centers (cell nuclei) in a homogeneous medium of slightly different index of refraction. The curve generated by subtracting the data from the two different polarization images can be mathematically correlated to the size of scattering particles within the background tissue thus enabling detection of cancer cells with enlarged nuclei when contrasted to normal lung tissue (see figure).
The HSIE produces in vivo 2-D images for perpendicular (upper left) and parallel (upper right) polarization orientations of detection versus illumination from the imaging probe. The spectral signatures (center) are shown in the point areas of choice (small rectangles on upper figures) and the oscillations of the perpendicular-minus-parallel spectral signals (bottom) are used to determine the size of the scattering particles, as predicted by Mie theory and its application to human tissue. This set of images comes from a patient’s bronchus.
In addition to the challenge of developing a mathematical model to correlate the spectral image data to the actual presence of cancerous tissue, practical considerations such as involuntary patient movement (near the heart, for example) must be considered. For now, the researchers have overcome this obstacle by inserting a baseline 610-nm optical scan at the beginning, middle, and end of the one-quarter-second wavelength acquisition set. Any significant change in intensity between these three 610-nm images within the quarter second required to acquire an image set is due solely to patient movement and requires a repeat scan until the intensity is stabilized.
While the scientists have made considerable progress in correlating the spectral image sets to tissue abnormalities, including precancerous conditions such as dysplasia, there is still considerable work to be done before this technology can be practically applied in a clinical setting to the simultaneous detection and treatment of cancer tissue.
REFERENCE
1. E. Lindsley, E. S. Wachman and D. L. Farkas, Progress in Biomedical Optics and Imaging 5, 11 (July 2004).