Optical Biosensors: Lasers look Alzheimer’s in the eye
Quasi-elastic light scattering and fluorescent ligand scanning on one platform may lead to earlier detection and improved management of Alzheimer’s and other neurodegenerative diseases.
Paul Hartung
In combating Alzheimer’s disease, researchers and clinicians have faced one constant factor that has proved a major challenge-the difficulty in diagnosing the disease, aggravated by numerous obstacles to accurate detection. Conventional approaches to diagnosing Alheimer’s and other neurodegenerative illnesses are either prohibitively expensive, marginally effective, or both-a challenge made more difficult because a commercial in vivo biomarker for Alzheimer’s disease has not been available.
One promising avenue, however, combines laser-based technologies with the discovery of a new biomarker for Alzheimer’s. In 2003, Lee Goldstein and his team at the Harvard Medical School (Boston, MA) reported that the same beta-amyloid aggregates (A-beta proteins) that create the signature plaques in the brains of Alzheimer’s patients, could also be found in the periphery of the lens and the aqueous humor of the eyes of those patients.1 Goldstein and his team found that detectable similarities between the proteins in the brain and in the eye could be measured noninvasively and tracked optically (see www.laserfocusworld.com/articles/241165).
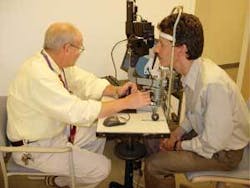
To make use of these findings in a clinical setting, the team developed two complementary technologies-quasi-elastic light scattering (QLS) and fluorescent ligand scanning (FLS)-to enable noninvasive quantitative measurements of amyloid aggregation in the lens of the eye. Neuroptix is commercializing these technologies and combining them on a single platform into a screening system designed for use in a doctor’s office (see Fig. 1).
Quasi-elastic light scattering
Quasi-elastic scattering-alternatively known as photon-correlation spectroscopy, laser Rayleigh scattering, dynamic light scattering, or self-beat spectroscopy-provides information about the diffusion coefficient of large molecules and small particles suspended in a fluid. Quasi-elastic light scattering is measured by focusing a laser beam in the fluid under investigation and collecting the light scattered at various angles from a small sample volume (typically a 0.100-mm-diameter sphere). The scattered light is detected by a photon-counting detector and correlator assembly to produce a correlation output function. Based on the Brownian motion of solute molecules within a solvent, QLS provides an estimate of the molecular size of scattering elements within a solvent system by precisely measuring the intensity of dynamically scattered light as a function of time.
In protein-condensation diseases such as age-related cataract, Alzheimer’s disease, Parkinson’s disease, and others, proteins associate to form very high-molecular-weight aggregates that are large enough to scatter incident light. By monitoring this light scattering, the process of pathogenic protein aggregation can be directly measured. To investigate the diagnostic potential of QLS, Goldstein’s research team aimed pulses of low-power IR laser light into designated zones of the eye (see Fig. 2). They measured the backscattered light using a photodetector and an autocorrelator assembly and obtained a highly precise and specific picture of the suspect proteins. The technique promises accurate monitoring of Alzheimer’s progression because operators can repeatedly examine the exact same region of the eye to detect changes in the size and shape of proteins over time.
If the molecules or particles under QLS investigation are all of the same size and in a dilute solution or suspension, the correlation function will exhibit an exponential decay, with a time constant that can be analyzed to estimate the hydrodynamic radius of the molecule or particle under investigation. The analysis used to derive the hydrodynamic radius is influenced by several parameters in addition to the decay rate of the correlation function. The wavelength of incident light, the index of refraction, viscosity, temperature of the fluid, and the angle at which the intensity of scattered light is measured are all factors that influence the output function.
If the molecules or particles have more than one size and/or are at a higher concentration, however, the correlation function will be more complex. Nevertheless, size information and other characterization information can be determined. For example, it is possible to determine virial coefficients (that characterize pair-wise interaction potential between particles) in nonassociating systems or association constants in binary systems that undergo specific dimerization (monomer linking) reactions.
To ensure that the suspect proteins are indeed Alzheimer’s A-beta proteins, Goldstein’s research team developed the follow-on FLS component of the screening test, which involves topical application of a beta-amyloid-specific small molecule to the patient’s eye. The molecule is absorbed into the lens and binds to the amyloid aggregates. The FLS system excites the fluorescent ligands that bind to amyloid and quantitatively measures emissions in specific anatomical locations to biochemically confirm the presence of amyloid.
The binding compounds emit light outside of the blue-green range, at wavelengths of 550 to 700 nm. If binding increases over time, a positive diagnosis can be made-and clinicians can monitor the progression of the disease by measuring levels of fluorescence. This might also enable them to detect and follow clinical response to drug treatment.
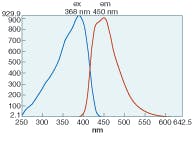
Neuroptix has licensed a full series of fluorescent ligands from the University of Pittsburgh (Pittsburgh, PA) and is developing them for human use. Two compounds, methoxy-X04 and methoxy-X34 bind strongly to amyloid and are highly fluorescent (see Fig. 3). Work has been previously published describing the binding characteristics of these compounds in ex vivo tests. The next step in the development process is to perform the toxicology studies required for testing in humans. To quantify the biochemical nature and the size of the aggregates, simultaneously, FLS is being integrated with QLS on the same platform.
Outlook
Given that Alzheimer’s diagnostic regimes are currently limited to invasive and/or unreliable tests, the need for an alternative, noninvasive “early warning system” that multiplies physicians’ (and patients’) options for intervention is clear.
Among existing diagnostic tools, none can provide a reliable early diagnosis: brain scans (magnetic resonance imaging and positron emission tomography) are expensive, and only work at the later stages of disease progression; cerebro-spinal fluid tests require a risky spinal tap and are not sensitive enough for definitive diagnosis; blood tests can only determine genetic predisposition in some cases; and urine tests are not sensitive enough and yield highly variable results.
An ideal diagnostic test would be predictive, reliable, inexpensive, and widely available, and fast and simple enough to be performed accurately by a general practitioner. The emerging combination of QLS and FLS technologies could potentially provide a simple screening test, similar to an optometrist’s slit lamp examination.
To date, preclinical results using QLS and FLS in separate studies indicate that the technology is capable of detecting early-stage amyloid pathology in the eye. Clinical prototypes featuring QLS capability have been built and tested, further supporting the idea of making in vivo measurements.
Quasi-elastic light-scattering instruments use a Class I laser product, only dimly visible to the human eye, which means that the instrument is not only well tolerated by patients, but is also safe. The combined platform, which merges both technologies and is currently under development for clinical studies, has attracted attention from the pharmaceutical community for testing purposes. There is a strong development pipeline of new therapeutic drugs for Alzheimer’s disease, and pharmaceutical companies need more-sensitive tools for titrating subjects entering clinical trials, for monitoring disease progression, and for measuring the effectiveness of therapies.
This combined platform is currently available in the United States for investigational use only, and is intended for clinical evaluation and measurement of aggregation in anatomically defined regions of the anterior segment of the eye. Using a measurement volume of 65 µm3, the analytic volume can be precisely positioned for reproducible and anatomically definable study. In addition to QLS and FLS in anatomically defined regions of the lens, the device is designed to be used as a clinical laser ophthalmoscope and for anterior segment photography.
REFERENCE
1. L.E. Goldstein et al., The Lancet 361(9385) 1258 (April 12, 2003).
PAUL HARTUNG is president and CEO of Neuroptix, 32 Jackson Drive, Acton, MA 01720. E-mail: [email protected]; www.neuroptix.com.