TERAHERTZ IMAGING: Terahertz methods show promise for breast cancer
New imaging tools based on terahertz technology aim to provide vital information during surgery on the full extent of disease.
VINCENT P. WALLACE
The introduction of stable, turnkey ultrafast lasers and improvements in the generation and detection of terahertz radiation have led to an explosion of interest in a variety of applications from pharmaceutical science, security screening, and medical imaging.
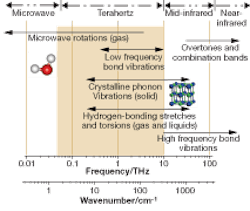
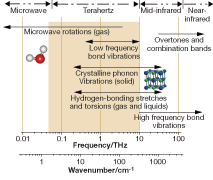
Terahertz time-domain spectroscopy and imaging were pioneered in the mid-1990s by Martin Nuss and coworkers at Lucent Technologies Bell Laboratories1 (Murray Hill, NJ; see “Efforts to generate terahertz date to the 19th century,” p. 85). Since then, many other research groups have successfully built terahertz systems (see Fig. 1).2
Early experiments by Arnone et al. demonstrated that terahertz pulsed imaging (TPI) could have medical applications-their terahertz images of porcine tissue showed contrast between muscle and fat.3 Mittleman et al. used terahertz imaging to assess burns in chicken breast.4 More recent work at TeraView used TPI to reveal contrast between regions of healthy skin and basal cell carcinoma, the most common form of skin cancer.3
TeraView is currently collaborating with surgeons and histopathologists to investigate the feasibility of using TPI to map tumor margins in freshly excised human breast tissue. With further development, we aim for the technology to be used during surgical procedures to enable complete removal of disease. Recent results have shown for the first time that TPI can reliably distinguish between normal breast, tumor, and even in situ disease (early stage cancers) in excised tissue samples.4 The finding opens up exciting new medical applications for terahertz technology (see “Terahertz offers medical benefits,”).
In the United Kingdom about 90% of breast cancer patients undergo surgery to treat the disease. Of these, slightly more than 50% will undergo breast-conserving surgery and the remainder mastectomy. In breast-conserving surgery, the primary tumor is removed with a margin of normal tissue around it. The surgeon uses intraoperative clinical palpation of the excised specimen and radiography to determine tumor margins.
Such procedures can underestimate the extent of tumor, however. So the excised specimen is then sent for routine histopathology, the “gold standard,” to determine whether the margins are free of malignancy, but this is a time- and labor-intensive process. And about 10% to 15% of patients require a second operation because histopathology reveals the margins involve cancer, thereby increasing the likelihood of recurrence.
There is a clinical need, therefore, to accurately define the margins of tumor during surgery to conserve normal tissue and minimize the number of second surgical procedures (which require additional hospital resources and increase the risk of patient morbidity). No current technology is available to do this.
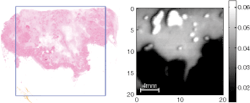
In our studies, several samples of excised breast tissue were imaged and parameters from the time-domain pulse were used to provide contrast. The size and shape of tumor regions in the terahertz images were compared with the corresponding histology section. Good correlation was found for area and shape of tumor in the terahertz images compared to that of histology (see Fig. 2).
We have also performed a spectroscopy study comparing the terahertz properties (absorption coefficient and refractive index) of excised normal breast tissue and breast tumor. Both the absorption coefficient and refractive index were higher for tissue that contained tumor. These changes are consistent with higher water content and structural changes, like increased cell and protein density. This study demonstrated the potential of TPI to image both invasive breast carcinomas and ductal carcinoma in situ, and encourages further work.
Terahertz system
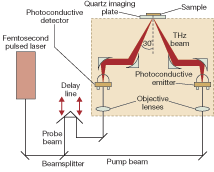
The TPI system used in our tissue studies uses a photoconductive emitter and a detector that measures the amplitude and phase of the transient electric field (see Fig. 3). This combination yields terahertz pulses with excellent signal-to-noise ratio (S/N) and high dynamic range.
The instrument generates pulses of broadband terahertz radiation in the range 0.05 to 4 THz with a spectral resolution of 0.03 THz. The broadband signal-to-noise ratio is typically around 4000:1. The optics are raster scanned in the x-y plane to collect a grid of pulses. The dataset is three-dimensional with time as the third axis. The sample is placed on the quartz imaging plate and a 2 cm2 area can be scanned in a few minutes.
Contrast mechanisms
Sources of contrast in tissues are not well understood and are being investigated; both TPS and TPI explore low-frequency torsional and vibrational motions in molecular systems. These motions are either flexing of the individual molecules or intermolecular interactions via strong hydrogen bonds or weaker van der Waals forces. These have been utilized to identify polymorphs of drugs.
The absorption spectrum of water exhibits a very strong, broad peak centered at 5.6 THz attributed to resonant stretching of the hydrogen bond between water molecules. The effect of this absorption peak, which extends down to the frequency range used in TPI and TPS, makes these techniques highly sensitive to water concentration. Thus, water absorption is evident in the terahertz properties measured for soft tissues, which explains the contrast seen between, for example, muscle and adipose tissue. More work is required to fully understand the contrast between diseased and healthy tissue.
REFERENCES
1. B.B. Hu, M.C. Nuss. Optics Lett. 20(16) 1716 (1995).
2. www-ece.rice.edu/~daniel/groups.html
3. D.D Arnone, C.M. Ciesla, S. Egusa et al, Proc. SPIE 3828, 209 (1999)
4. D.M. Mittleman, M. Gupta, R. Neelamani et al., Appl. Phys. B. 68, 1085 (1999)
5. V.P. Wallace, A.J. Fitzgerald, S. Shankar et al., British J. Dermatology 151, 424 (2004).
6. A.J. Fitzgerald, V.P. Wallace, M. Jimenez-Linan, et al., Radiology 239, 533 (2006).
VINCENT P. WALLACE is head of medical applications at TeraView, Cambridge, England; e-mail: [email protected]; www.teraview.com.
Terahertz offers medical benefits
Terahertz radiation covers frequencies from 100 GHz to 10 THz and offers potential benefits as a medical imaging modality. These include:
■ Terahertz radiation is nonionizing (that is, it is safe)
■ It can penetrate epithelial tissues
■ There is less scatter than IR/optical due to longer wavelength so images at depth do not suffer from “blurring”
■ Wavelengths are short enough for submillimeter resolution: typically 250 µm laterally, 20 µm axially
■ Time-domain imaging uses amplitude and phase information in reflection or transmission measurements and can be used to generate 3-D images
■ Terahertz is an established tool for physical and chemical analysis
■ Photoconductive systems can provide broadband spectroscopic information from 100 GHz to 4 THz
Efforts to generate terahertz date to the 19th century
Efforts to extend optical methods to generate terahertz date back to measurements of blackbody radiation using a bolometer by Heinrich Reubens and Ernest Fox Nichols in the 1890s.1 Over three-quarters of a century later, in 1975, David Auston at AT&T Bell Laboratories developed a photoconductive emitter that accelerated progress toward bridging the terahertz gap.2
The photoconductive emitter consists of a small piece of semiconductor crystal (commonly gallium arsenide), on which two planar metal electrodes, whose geometric design is that of an antenna, support a large electric field across its surface.
Ultrafast (approximately 100 fs) pulses of light (commonly from a Ti:sapphire laser at a wavelength of 800 nm) are then focused onto the gap between the electrodes; conveniently the photon energy of the 800 nm light is above the bandgap of the gallium arsenide semiconductor. The photo-generated electron-hole pairs excited near the crystal surface rapidly change the conductance.
Application of a bias accelerates the charge carriers and leads to a rapid change in the current density. The changing dipole produces a terahertz transient in the antenna that is radiated into free space. The shape of the pulse and hence the frequency distribution depend on the design of the antenna and the carrier dynamics. Several techniques can be used, from bolometric measurement and electro-optic sampling to photoconductive receivers, the latter essentially operate in reverse to photoconductive emitters.
REFERENCES
1. E.F. Nichols. Physics Rev. 4, 297 (1897).
2. D.H. Auston. Appl. Phys. Lett. 26(3) 101 (1975).