BIOMEDICAL OPTICS: Lasers, ultrasound let doctors eavesdrop on skin cancer
Melanoma is one of the most vicious forms of cancer; if not detected and removed in its earliest stages, it will penetrate into the deep layers of the skin, then pass into the circulatory and lymphatic systems, spreading to other organs. According to the American Cancer Society, the five-year survival rate for Stage 1 malignant melanoma (where the cancer is still in the epidermis) is 90% to 95%, versus 18% for Stage 4 (where the cancer has penetrated to the lower dermis).
Researchers at the University of Missouri (Columbia, MO) have shown that by using photoacoustic detection, which combines pulsed laser energy with ultrasound, they can detect the spread of skin-cancer cells in the blood by listening to the sound of the melanoma cells as they “explode” after being stimulated by the laser pulses.1
In laboratory tests, the Missouri-Columbia team was able to detect melanoma cells obtained from actual patients, detecting as few as 10 cells in the saline solution. The dark, microscopic granules of melanin in the melanoma cancer cells absorb 5 ns pulses of 450 nm light from a frequency-tripled Nd:YAG laser pumping an optical parametric oscillator. The cells go through rapid cycles of expansion as they heat up and shrinkage as they cool down; these sudden changes generate acoustic waves.
Other human cells do not contain pigments with the same color as melanin, so the melanin signature is easy to tell apart from other noises, said John Viator, assistant professor of biological engineering and co-author of the study. And the presence of melanin granules in the blood is an unmistakable sign, he added, because only melanoma causes melanin to appear in the human blood.
“Melanin is a broadband optical absorber that absorbs all colors of light well into the red, and melanoma cells are mutants of normal melanocytes that still produce melanin,” Viator said. “When they break off from the original skin lesion and go into the blood stream, they have this melanin. When we extract a blood sample and spin it out, there are layers, and somewhere in the middle is the mononuclear layer, which contains the white cells and melanoma cells, and in that mononuclear cell layer you should only have things that have no color. So if there is melanin in there, there is color and thus sound.”
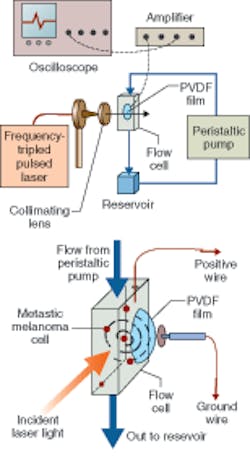
In addition to the laser, a quartz flow cell was used as an excitation and acoustic-wave collection device; the light beam was collimated before entering the flow cell using two plano-convex lenses (see figure). The acoustic waves were detected by a 100 µm piezoelectric polyvinylidene difluoride copolymer film sealed on the lateral surface of a 5-mm-diameter detection aperture of the flow cell and changed into a voltage signal displayed by a 200 MHz oscilloscope triggered by a photodiode. The signals were amplified with a gain of 25 via a 350 MHz amplifier. Latex microspheres with a diameter of 6.6 µm were used as melanoma tissue phantoms to calibrate the system.
Detecting metastasis
This new blood test would enable physicians to have a more accurate method of monitoring for metastasis. In fact, the blood-test procedure could be performed regularly such as in screenings for high-risk patients, requiring just a small sample of blood, and its results would be almost immediate—just 30 minutes, according to Viator.
The team is now planning a pilot study on actual blood samples from patients; larger clinical studies will need to be done, but the test shows great promise for early detection of the spread of this disease, according to Viator. He and his colleagues are also working with other Missouri-Columbia scientists in the veterinary college and the department of surgery to extend the reach of its technique to other types of cancer by introducing artificial materials to act as light absorbers. For example, gold nanoparticles could be attached to the cells using proteins that bind to special receptors on the cells’ membranes. With their own photoacoustic signature, the gold particles would then signal the presence of cancer cells.
REFERENCE
1. R.M. Weight et al., Optics Lett. 31(20) (Oct. 15, 2006).
About the Author
Kathy Kincade
Contributing Editor
Kathy Kincade is the founding editor of BioOptics World and a veteran reporter on optical technologies for biomedicine. She also served as the editor-in-chief of DrBicuspid.com, a web portal for dental professionals.