Neuroscience/Bioimaging: Optomechanics reveals transmitter roles in neuromuscular junctions
A comprehensive understanding of how the central nervous system communicates with and controls muscle function requires unraveling the precise roles of neurotransmitters—the chemical signaling agents that allow a cell to influence activity in another cell without direct electrical connection. But despite there being more than 200 varieties now identified, our understanding of neurotransmitters is incomplete.
A team led by Paul Brehm at the Vollum Institute at Oregon Health and Science University (OHSU, Portland, OR) is using a variety of microscopy techniques to change this by investigating neuromuscular signaling in zebrafish embryos. The researchers combine electrophysiological measurements with differential interference contrast (DIC) and fluorescence imaging, and sometimes optogenetics, in a microscope setup that depends on drift-free precision probe motion. By working with zebrafish mutants, they have correlated certain transmitter deficiencies with several identified human disorders.
Microscopy using zebrafish
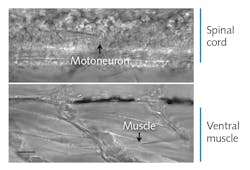
Zebrafish embryos are model organisms with the functional and morphological hallmarks of mature vertebrate synapses, and their optical transparency makes spinal cord neurons and muscle cells amenable to visual identification and direct recording. “To date, this tiny organism represents the only opportunity to stimulate single neurons in the spinal cord of a vertebrate animal and to record the resultant activity in target muscle cells,” Brehm said (see Fig. 1). In these embryos, “each neuron communicates only with location-specific muscles and each muscle cell is paired with only one of the four primary spinal neurons.”
To analyze neuromuscular function, the group uses electrophysiology (patch clamp technology) in the form of “whole cell” recording. The cells are imaged and selected based on DIC microscopy, and sometimes cell-specific fluorescence markers (e.g., green fluorescent protein, or GFP) and calcium fluorescence imaging.
As part of paired recording work, the team creates and uses a host of zebrafish mutants—many have a single defective gene that leaves them unable to release or respond to a specific neurotransmitter. Even severe disorders that may cause embryonic mortality in mammals can be studied in the zebrafish embryo.
Precision control for recording
In patch clamp recording, a flat-tipped micropipette serves as both a recording and stimulating electrode, and it interacts with a cell (or parts thereof) in various ways. The team’s whole-cell approach involves sealing the glass tip to the cell membrane and then penetrating the outer membrane. This results in dialysis of the internal cell contents with the patch pipette solution and a common electrical connection between the amplifier and the inside of the cell. It is critical that the insertion creates a high impedance seal between the pipette glass and the membrane, electrically isolating the contents from the surrounding bath solution. This allows measurement of the potential between the intra- and extra-cellular contents. The entire experiment is conducted in a Faraday cage to eliminate ambient electrical noise (see Fig. 2).The recording chamber is located in a novel optomechanical setup that the team spent years perfecting. This setup is built around a Zeiss upright microscope and Siskiyou micromanipulators. The microscope has a 40X water immersion objective and a long working distance to accommodate two or more electrodes. The chamber is mounted on a platform with an adjustable post, allowing the specimen to be held in a fixed position.
The researchers expose the spinal cord, with each hemi-segment containing one caudal primary (CaP) motor neuron that they visualize and identify using DIC microscopy (see Fig. 1) and sometimes GFP.
The critical mechanics of making the electrical seal with the target neuron can be understood from Figure 3. The pipette electrode, which has a 2-µm-diameter tip, is mounted on a four-axis (x, y, and z, plus tilt) micromanipulator assembly. The team tried several commercial models before selecting the MX 7600 (Siskiyou), which uses DC servo actuators driving crossed-roller bearing stages. “Steppers and piezo systems are problematic since they have a tendency to step in increments that make fine moving difficult,” said Brehm, adding that “Hydraulics tend to drift, particularly as they age,” which is also problematic. But “DC servos draw no current when stationary, and the Siskiyou units do not exhibit the recoil/backlash often found with other DC servos,” he explained. He also notes that a controller with a rotary knob (Siskiyou MC1000e-R/T) gives the responsive feel of a direct, fine-resolution motion screw rather than a digital indirect interface.The 30° angle of approach is critical to clear the objective and allow penetration of the elastic membrane surrounding the spinal cord, and a particular trajectory will allow proper contact with the specific motoneuron cell body. While bringing the pipette towards the CaP neuron, the researcher keeps it under slight positive pressure to avoid debris collection on the tip. As contact is made, fine control over advancement of the tip is critical. When the membrane is indented properly, the abrupt removal of excess positive pressure causes it to rebound and to form the so-called gigaseal (resistance between the inside of the electrode and the outside >109 Ω). The pipette is then changed to negative pressure, pulling on the membrane and rupturing it.
For paired recording, the two electrodes are located on neurons and muscle that are separated far outside the tiny field of view. For this reason, the microscope is completely mechanically separate from the fixed-position recording chamber and manipulators. As shown in Figure 2, the Zeiss traveling microscope is mounted on a motion stage (Siskiyou MXMS-115cri) powered by DC servo actuators to avoid electrical noise during recording. The joystick controller provides true diagonal motion, as opposed to motion synthesized from x, y stepping. The two micromanipulator systems and the recording chamber are directly mounted on an optical table supporting the entire apparatus.
To set up the second electrode, the researcher relocates the microscope to the ventral muscle using the joystick and chooses a fast muscle cell from the target field. Again, pressure and pipette motion are combined to create a seal. The researcher now has an arrangement that allows stimulation of the motoneuron using native firing patterns underlying swimming, while simultaneously recording the synaptic responses (see Fig. 4). Optical fluorescence detectors of synaptic transmission can provide dynamic activity data from the muscle cells.Having perfected their system and defined experiment protocols, the OHSU group constructed several copies of their setup and recorded a detailed video (http://bit.ly/PatchClamp) to help other labs make similar measurements.
Insight into human syndromes
Because this research involves studying fundamental unitary events in neuromuscular transmission, it is providing important insights applicable to neuromuscular function and dysfunctions in higher vertebrates, specifically humans, where mutational disorders are collectively called myasthenic syndromes. An example is rapsyn-deficient myasthenic syndrome, which in humans is characterized by weakness in voluntary muscle contraction, a direct consequence of greatly reduced synaptic responses that result from failure of acetylcholine receptors to cluster at the point of contact between the motoneuron and muscle. As with other myasthenic syndromes, the general muscle weakness is accompanied by use-dependent fatigue, which has been poorly understood.
The OHSU group was able to investigate the underlying cause of this fatigue through use of paired patch-clamp recordings from a rapsyn-deficient mutant line of zebrafish. They found that as stimulation frequency is increased in the motoneuron, both wild-type and mutant neuromuscular junctions depress to steady-state response levels—but the latter shows exaggerated depression. Analysis of the steady-state transmission revealed that vesicle reloading and release at individual release sites is significantly slower in mutant fish during high-frequency activities. This pointed to a combination of reductions in postsynaptic receptor density and slowed presynaptic release that collectively serve to reduce synaptic strength to levels below the threshold for muscle action potential generation, thus accounting for use-dependent fatigue in sufferers of this myasthenic syndrome.
The ability to interrogate neuron-neuron and neuromuscular signaling at single-cell resolution has revolutionized our understanding of neuroscience. Such experiments depend on the ability to mechanically manipulate probes—be they tightly focused laser beams or humble micropipettes—near the limits of microscope resolution.
About the Author
John Wingerd
Senior Mechanical Engineer, Siskiyou Corporation
John Wingerd is senior mechanical engineer with Siskiyou Corporation (Grants Pass, OR).