By Stratos Kehayas, PhD
Photonics Division President, G&H Group
Advances in life science have progressed in applications as diverse as medical imaging, therapeutics, and analytics. Yet if there is a common thread linking all these frontiers: it is a marked increase in precision fueled by developments in photonic technology.
Consider emerging advancements such as the ability to visualize subcellular dynamics, guide surgical instruments with micron-level accuracy, or analyze vascular pathologies in real time. Though these developments are unfolding across vastly different clinical and research domains, they share a common foundation: Continuing advances in photonic components and systems are enabling life science researchers and medical professionals to manipulate, transmit, and detect light with unprecedented control.
Life sciences stand to benefit as photonics suppliers continue to sharpen wavelength selectivity, energy efficiency, and signal integrity. Several high-growth sectors today exemplify where photonic-enabled precision is fueling recent advances, including fluorescence microscopy, flow cytometry, robotic-guided laser surgery, optical coherent tomography (OCT), and others.
Fluorescence Microscopy and Flow Cytometry: The Information Density Challenge
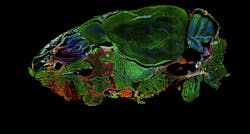
Widefield microscope image of a DAPI-stained mouse head. Sagittal plane: 4x, 0.2 NA. Courtesy of University of Applied Sciences Biberach (https://p-inst.com/life-science/)
The drive toward multiparametric analysis has fundamentally changed the performance envelope for wavelength manipulation technologies. Where early flow cytometry systems could simultaneously distinguish two or three cell populations, researchers now routinely deploy panels that can detect over 20 parameters — and the trajectory of recent developments points toward 40+ parameter systems. The increasing information density from multiparametric flow cytometry systems creates a cascade of photonic challenges: More fluorophores mean tighter spectral spacing, which demands sharper wavelength selectivity, and the need for faster switching between detection channels.
Although generating the excitation light is not trivial, laser sources have kept pace with these demands. The challenge lies in everything that happens afterward, including deflecting the beams to precise spatial positions, modulating intensity with microsecond response times, and filtering detection paths to isolate signals within an increasingly crowded spectral space.
Acousto-optic devices are starting to become essential tools by enabling dynamic beam control without mechanical motion. In spatial flow cytometry, for example, acousto-optic deflectors allow separate multiple interrogation points along a sample’s flow stream. This effectively creates parallel analysis channels from a single laser source, enabling higher throughput without sacrificing measurement precision.
Tunable filters present related challenges. As researchers pack more fluorophores into single assays, spectral unmixing becomes critical to accuracy. The ability to rapidly tune detection wavelengths while maintaining sharp spectral edges determines how well the closely spaced emission peaks can be resolved. Access to specific wavelength bands depends on the optical properties of acousto-optic crystals and filter substrates. Vertical integration in material supply chains is a powerful advantage and can allow a manufacturer to control crystal quality and optical characteristics, enabling devices that operate at wavelengths matched to emerging fluorophore chemistries.
These wavelength manipulation capabilities range from acousto-optic beam control to spectrally precise tunable filters and enable applications such as high-content screening — where pharmaceutical researchers need to extract multiple readouts from single cells — and in clinical diagnostics, where rare cell detection requires high specificity and high throughput. Wavelength control, not biology, defines how many parameters researchers can reliably multiplex.
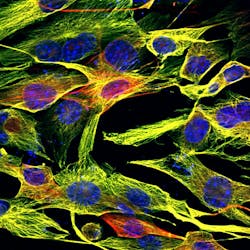
Stained mouse fibroblasts imaged with multiphoton microscopy at 60x, 1.4 NA, with a 160-µm field of view. Courtesy of University Konstanz. (https://p-inst.com/life-science/)
Lasers + Robotics = Precision Surgery
Surgical precision in a growing number of medical procedures increasingly depends on how quickly and accurately photonic components can translate surgeon intent into robotic action. Whether guiding ablation beams through corneal tissue or providing optical tools for robotic positioning, these photonic systems must operate with clinical-grade reliability.
The eye is an extremely sensitive and delicate organ with complex millimeter and sub-millimeter features and interfaces. The success of procedures in the eye depends on creating a precise laser beam that often is also required to be steered with micron-level accuracy. This system could be either part of therapeutic surgery (e.g. treating post cataract ) or as a preventive tool to detect underlying eye conditions. Acousto-optic deflectors enable such precision by translating digital control signals into physical beam positioning faster and more repeatably than mechanical galvanometers can. These component technologies enable millions of annual procedures with predictably positive outcomes.
Robotic surgical platforms represent a more complex integration challenge. These systems combine multi-arm coordination, tremor reduction, and enhanced visualization to enable smaller incisions, reduced blood loss, and faster patient recovery. The photonic infrastructure supporting robotic surgery includes advanced fiber optics for endoscopy, imaging, and illumination. While the specific implementations remain proprietary, the underlying principle is clear: Robotic precision requires low-latency optical feedback to coordinate motion across multiple degrees of freedom.
Robotic surgery platforms have moved from specialized academic centers to widespread clinical deployment, driven by improvements in accuracy and reduced complication rates. As these systems evolve, real-time micron-scale positioning will demand extremely low latency feedback controls, while vision-guided systems will require higher bandwidth. And the clinical environment will demand reliability that doesn’t degrade over thousands of cycles.
The integration of artificial intelligence (AI) with surgical vision systems promises to further amplify these capabilities. Photonic sensors will be able to feed data into algorithms that can assist with navigation, identify anatomical structures, or even provide decision support.
OCT: Beyond Ophthalmology
OCT enables cardiologists to assess stent deployment quality and vessel wall characteristics with micron-scale resolution from within the artery itself. The level of detail, impossible with conventional angiography, depends on fiber optic couplers that deliver both the bandwidth for deep tissue penetration and the spectral flatness required to maintain consistent resolution throughout the vessel wall.
OCT might offer the clearest example of how component-level advances translate directly into new clinical capabilities. Although advances in detector sensitivity and computational power have driven OCT’s evolution from ophthalmology to cardiology to gastroenterology, fiber optic couplers and laser diodes have been equally critical.
OCT has become the standard of care for retinal disease diagnosis, providing micron-scale cross-sectional images that enable early detection of pathological changes before they impact vision. But OCT is seeing new growth as it branches out into new anatomical territories where cross-sectional imaging provides clinical insights impossible with surface-only techniques.
Interventional cardiology represents the most technically demanding expansion. Here, OCT systems must integrate with narrow catheter lumens to image arterial walls from within blood vessels. The procedure uses a rotating optical probe that withdraws through the artery while acquiring images at rates fast enough to capture vessel topology before blood flow obscures the view. This enables capabilities beyond conventional angiography, such as the assessment of stent placement and vessel wall composition, as well as the identification of plaque type.
The technique requires both adequate resolution and sufficient penetration depth, which makes two parameters of key importance: Fiber coupler bandwidth and spectral flatness.
Broader coupler bandwidth enables deeper tissue penetration, which is critical for imaging through vessel walls up to several millimeters thick. Spectral flatness, in turn, ensures axial resolution remains consistent across the imaging depth, preventing the image quality degradation that occurs when coupler response varies with wavelength.
What matters clinically is that these photonic parameters directly determine diagnostic capability. A coupler with insufficient bandwidth limits how deeply a cardiologist can image into vessel walls. Spectral nonuniformity degrades resolution at the exact depths where pathology often resides. Component performance isn’t just a technical specification; it’s a clinical constraint.
The multipliers: Quantum Sensing and AI
Exemplary image of a photonic laser engine that typically includes broadband couplers, optical delay lines, interferometric structures and polarization diverse receivers in order to enable high resolution OCT scans.
Courtesy of G&H.
The three frontiers above represent established applications where photonic precision drives clinical and research value. Two emerging technologies promise to amplify this impact further — one at the component level, one at the system level.
Quantum sensing represents a new frontier in measurement sensitivity. Fiber-coupled acousto-optic modulators combined with fiber-coupled collimators enable the precise laser controls required for atom trapping and cooling. These two techniques can achieve sensitivities that are orders of magnitude beyond conventional optical sensors. While applications in atomic clocks and precision navigation are established, biosensing implementations remain in development. The question is how rapidly these quantum measurement capabilities can translate into clinical tools for detecting biomarkers, imaging neural activity, or characterizing tissue at molecular scales.
AI’s role as an amplifier takes a different form. It enables life science users to extract more value from the data that photonic systems already capture. In mammography, for example, AI algorithms can now analyze optically captured images to detect precancerous patterns that radiologists might miss. In cardiology, AI processes OCT spectroscopic data to assess plaque composition and mobility in real time to provide insights beyond visual interpretation. In surgical robotics, machine learning algorithms can assist navigation and support decisions based on machine vision data. The pattern is consistent across applications: Photonics provides the high-quality measurement modality, while AI multiplies its clinical value.
The next generation of life science instrumentation will depend on continued advances in wavelength control, energy/power efficiency, and signal integrity. But as quantum sensing and AI mature from research tools to clinical deployment, the potential value that photonic systems offer life science will only multiply. Precision isn’t just a feature of these systems; it is the foundation on which they are built.