Microscopy combo enhances super-resolution imaging
Traditional super-resolution microscopy methods do not typically present high-enough resolution of all three angles of a 3D structure when working at a nanometer scale. Yet, by combining two different imaging techniques, researchers have achieved equal, consistent resolution across all three dimensions.
Developed in collaboration by researchers from University of Göttingen (Germany), Julius-Maximilians University of Würzburg (Germany), and the National Cancer Institute (Bethesda, MD), the new method brings together metal-induced energy transfer (MIET) imaging and a specific single-molecule localization microscopy (SMLM) technique called direct stochastic optical reconstruction microscopy (dSTORM).
Conventional microscopy techniques lack coherent resolution among three dimensions. This new combo method—detailed in a study published in Science Advances—alleviates the long-existing challenge of attaining the same super-resolution along the optical axis as it does laterally.
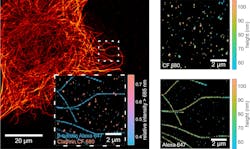
dSTORM delivers nanometer resolution laterally, while MIET imaging—a technique created by Göttingen physics professor Jörg Enderlein’s group in the school’s Biophysics Institute—produces powerful nanometer resolution along the optical axis of a structure. With this combination, isotropic 3D super-resolution fluorescence microscopy on the nanometer scale is now possible for resolving individual molecular complexes and structures (e.g., single hollow microtubules and clathrin-coated pits, which are proteins) on a length scale of <150 nm.
MIET is among a class of relatively simple imaging methods that uses near-field effects for achieving a similar resolution in SMLM as interferometric approaches. Other methods in that class—variable-angle total internal reflection fluorescence (TIRF) microscopy, which needs the evanescent/near-field above a cover slide for fluorescence excitation, and supercritical angle fluorescence (SAF) microscopy, which uses the near-field coupling of fluorescent emitters into optically denser cover slide glass—are also possible approaches for such imaging, but not as easy to implement as MIET.
“The core idea is to use the effect of fluorescence quenching near a metal layer,” Enderlein says. MIET uses the near-field coupling of fluorescent emitters to surface plasmons in a thin metal film deposited on a cover slide.
“The closer an emitter is to a metallized surface, the shorter its lifetime,” Enderlein says. “By measuring the lifetime of an emitter, we can convert this value into a distance from the metal (time-to-distance conversion).”
One microscope required
This technique’s relative simplicity sets it apart from others—it requires just a single-molecule sensitive fluorescence lifetime imaging microscope (FLIM). The only other requirements are a metallized substrate (cover slide with a 10–20 nm gold layer) and the software for the lifetime-to-distance conversion (see Fig. 1).
The MIET-SMLM/dSTORM technique reaches a 3D resolution that could nearly rival electron microscopy, which has a very high range of magnification and very high resolution (see Fig. 2). But the new method still needs fluorescent labeling, according to Enderlein, which poses a big challenge: Developing labeling strategies that bring the labels as close as possible to the target site of interest (e.g., small linkers and nanobodies).“We hope our method will find broad applications for resolving molecular complexes, such as multi-protein complexes, protein-DNA complexes, lipid membrane structures, etc.,” Enderlein says. “Now, with optical microscopy, we are really approaching the molecular-length scale of spatial resolution.”
About the Author
Justine Murphy
Multimedia Director, Digital Infrastructure
Justine Murphy is the multimedia director for Endeavor Business Media's Digital Infrastructure Group. She is a multiple award-winning writer and editor with more 20 years of experience in newspaper publishing as well as public relations, marketing, and communications. For nearly 10 years, she has covered all facets of the optics and photonics industry as an editor, writer, web news anchor, and podcast host for an internationally reaching magazine publishing company. Her work has earned accolades from the New England Press Association as well as the SIIA/Jesse H. Neal Awards. She received a B.A. from the Massachusetts College of Liberal Arts.