For polymer switches, tailored synthesis meets the reliability challenge
Polymer optical devices usually struggle for acceptance in the reliability-conscious telecommunications sector. But two key properties of polymers—their thermo-optic and electro-optic functions—continue to hold the promise of low-cost, high-performance devices. Measured against alternative material technologies that can offer these effects, polymer technology has a clear lead. This advantage continues to drive R&D in startup companies surviving the current downturn, and there is a growing acceptance of polymers as future partial solutions to bandwidth bottlenecks and system configuration problems.
How can polymeric materials, crudely labeled "plastics," be of serious consideration for high-performance and high-reliability optical telecommunications devices? The answer is that in some niche applications they may be the only option. For example, in applications in which lower cost and higher performance must be fused, such as optical waveguide switches and modulators, polymers can outperform rival technologies.
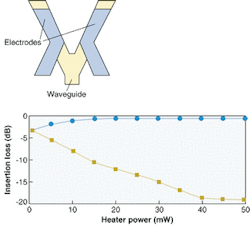
FIGURE 1. In a digital thermo-optic-switch waveguide structure with resistive heater electrodes, applying heat to one waveguide arm reduces the refractive index; input light will then emerge solely from the other arm (top). The switching characteristics of the switch are shown by squares, which indicate the optical output from the heated waveguide branch, and circles, indicating the unheated branch. Note that the device enters a region (above 45 mW) where the power-transfer characteristics are insensitive to external fluctuations.
The fact is, without some special attention, polymers do not have aspects of stability that would make them useful for universal deployment in the telecom sector. After most kinds of polymer material processing, residual stresses cause a slow aging of the initial optical and mechanical properties.
Polymers also have a propensity to absorb water and many undergo dramatic physical transitions at temperatures not unknown in deployed telecom systems. If this weren't enough, they are not normally transparent enough at the telecom infrared wavelengths, and in any case can exhibit unwanted optical birefringence as thin films.
To tackle these issues, researchers must have a strong belief in the potential for polymers, coupled with an unsatisfied market demand. But these drivers exist. Despite the telecom slowdown, there are still at least two areas of device functionality—the thermo-optic and electro-optic effects—that make a strong case for polymers.
Thermo-optic effects
"One man's meat is another man's poison"—and the thermo-optic effect in polymers is either a scourge or a godsend. Compared to inorganic glasses, the refractive-index change with respect to temperature (dn/dt) in polymers is more than one order of magnitude larger. Typical values hover above -10-4 K-1 and the negative sign hints at the underlying mechanism. On heating, thermal expansion dominates the refractive index change, increasing the fractional free volume (unoccupied space) and thus lowering the density.
Such large changes would increase the burden of thermal control of packaged devices and would disadvantage temperature-sensitive polymer devices such as directional couplers and interferometers. However, turned to an advantage, the large thermo-optic coefficient makes possible switches whose low switching-power operation far surpasses rivals.
A polymer-based digital optical switch that can be used for protection routing relies on large index changes to actively reconfigure the input-mode evolution properties and thus redirect the output power.1 Such a device achieves a stable operating condition even if there are ambient temperature fluctuations. Such insensitivity is a sought-after property. As if to emphasize the advantage of polymers, they also have low thermal conductivity. Applied heat stays where it is needed and less energy is required to produce it.
Electro-optic effects
Achieving faster data transmission not only means packing more wavelengths per fiber (as in wavelength-division multiplexing) but also in running each wavelength at a higher bit rate. Direct modulation of source lasers only goes so far without introducing spectral distortion (chirp). External modulators remove this problem by modulating a continuous-wave laser beam. But how fast can these devices go?
In the case of a traveling microwave design, the answer with polymers is: as fast as the drive electronics addressing it. Recent demonstrations of a polymer modulator show the -3-dB bandwidth to be around 150 GHz. Next-generation optical time-division multiplexing, by contrast, is set for 40 Gbit/s, leaving plenty of bandwidth capacity in the polymer device. The secret lies in the low dielectric constants of polymers. This fundamental property allows velocity matching, otherwise known as the equalization of the speeds of optical and microwave signals. No other electro-optic material class can equal this performance.
It's true that polymers are not generally considered interesting for electro-optic effects, but they can be made interesting if polymer chemists, physicists, and device engineers join forces. Electro-optic polymers are custom synthesized and processed. They must contain a high density of molecules with a large first nonlinear optical coefficient.
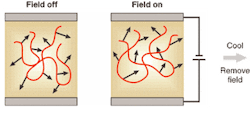
This property produces a molecular source of electro-optic modulation, but for a bulk effect the molecules must, on average, point in the same direction. Each one should have a dipole moment, µ, and if an electric field, E, is applied, while heating the polymer, such an alignment can be achieved. The torque (µ × E) exerted on the dipoles pulls them around to align with the field (see Fig. 2). Extraordinary electro-optic coefficients, r, can be obtained using this "poling" procedure. Values up to almost 100 pm/V have been shown, and since device drive voltage is inversely proportional to r, there is the chance of subvolt operation—and high bandwidth.2
Reliability and peripheral issues
Getting this performance advantage, however, often feels like only the first step. Look more deeply at the problem and a complicated set of material requirements emerges, each of which needs to be satisfied simultaneously.3
First let's look at the primary structure of a polymer. The polymer chains comprise atoms linked by covalent bonds: carbon-carbon, carbon-hydrogen, carbon-oxygen, and so on. It is not so much the strength of these bonds that is of concern but the frequencies at which they vibrate that has a bearing on performance in telecom devices. The fundamental frequencies of oscillation can be observed using infrared spectroscopy (wavelengths between 3 and 20 µm) where strong resonant absorption is seen, but the overtones of these anharmonic oscillators spill into the telecom ITU bands between 1.3 and 1.7 µm. Here they provide a significant mechanism for optical waveguide loss.
The trick is to increase the reduced mass of the oscillator; in other words, replace light atoms (hydrogen) with heavier atoms (fluorine) and thus lower the frequency of the fundamental oscillation. This change places higher-order overtones in the ITU region, and these overtones have reduced absorption strength. Thus, many commercially developed polymers are perfluorinated versions of their hydrocarbon analogs. Thrown in with this advantage, fluorinated polymers are highly resistant to moisture uptake and more thermally and chemically stable.
What about the secondary and tertiary structure? Polymer chains fold around each other like spaghetti on a plate, relying on weak van der Waals forces and perhaps hydrogen bonds to hold it all together. Nevertheless, many are described as "glassy" at useful temperatures and only exhibit rubbery behavior above the "glass-to-rubber" transition temperature, Tg.
These glassy polymers, unless modified, carry the baggage of their thermal history with them. Their fractional free volume is not constant but slowly reduces over time. Polymers that have been taken above Tg for some reason (maybe films deposited by solution spin coating, or those "poled" to get an electro-optic effect) are on a long, slow relaxation path that usually is of no practical consequence. If the device is to be used across a reasonably wide temperature range, however, (continuous operation at 70°C, for example) it must not exhibit any aging phenomena such as refractive-index or electro-optic drift, which can be a consequence of thermal relaxation.
The obvious answer is cross-linking. Tie the chains together using covalent bonds to form a three-dimensional network or "resin." Alternatively, try bonding the active molecule into the main polymer chain rather than hanging it off peripheral polymer chains. Or, perhaps best of all, use the active molecule itself as the cross-linking group. The effectiveness of these approaches is readily seen in electro-optic polymers in which the relaxation in electro-optic effect over time provides a convenient measure of stability. It is now common to find reports of indefinite stability of electro-optic response at temperatures close to 80°C or so.
Finally, with regard to the waveguide film properties themselves, aside from absorption loss, the polymer must form films in which scattering loss is minimized and preferably in which birefringence is minimal. Fortunately, the structural irregularity in the polymer chains most widely used produces fully amorphous, rather than partially crystalline, polymers. Thus, lightwaves see a homogeneous medium when propagating through the device. However, some of these problems still sometimes haunt the interdisciplinary teams whose job it is to advance this field. After poling and curing of electro-optic polymers, for instance, sometimes there is an increase in propagation loss due to damage introduced when the polymer is still "soft." Not impossible to overcome, but yet another issue to add to the list.
The large chemistry set now available has enough in it to answer some of the most stringent material questions that commercial reliability poses to the use of polymers in the telecom field. The story may not end with these advances. One further step may transform the components industry—plastic molding. Is it possible to combine this final feature with the high-specification materials already showing promise? Workers at Bell Laboratories (Holmdel, NJ) asked a similar question in 1974.4 Occasionally the problem is revisited, but for the moment, the dream of the $1 modulator remains just that.
REFERENCES
- M. B. J. Diemeer, Optical Materials 9, 192, (1998)
- Y. Shi et al, Science 288, 119, (2000).
- G. H. Cross, RAPRA Review Report 13(9) 153, (2002).
- G. D. Aumiller et al, J. Appl.Phys. 45, 4557, (1974).
GRAHAM CROSS is a lecturer in the Department of Physics in the University of Durham, Department of Physics, South Road, Durham DH1 3LE UK; e-mail: [email protected]