The SPIE Biomedical Optics symposium (BiOS; San Jose, CA, Jan. 20-25) has long been known for showcasing cutting-edge applications of lasers, optics, and optoelectronics in medicine. Over the years this meeting has served as a barometer of how the field is evolving and what the medical world can expect to see in terms of practical tools and applications in the near future.
Throughout the 1990s, the BiOS Symposium was devoted to the use of lasers for therapeutic applications in urology, ophthalmology, otolaryngology, photodynamic therapy, tissue welding, dentistry, and dermatology—in essence, the major medical disciplines. In the last few years, however, the trend at this meeting has been decidedly toward optical imaging and the physics behind it. Nowhere was this more evident than at the Saturday night “Hot Topics” session, an always well-attended event that provides a peek at the latest innovations in this field. At this year’s Hot Topics, invited speakers were given the opportunity to describe their latest research; many of the presentations focused on the use of infrared (IR) and near-infrared (NIR) imaging to optimize drug delivery and treatment.
The evening began with Thomas Baer of Stanford Photonics Research Center (Stanford, CA), who talked about the immense potential for photonic tools in cancer screening and diagnosis, such as combining optical techniques with high-resolution computed tomography for better detection of lung cancer. The challenge is how to better distinguish benign from malignant lesions, and multimodal imaging offers many advantages in this regard; for example, using optical techniques as an adjunct to CT to evaluate parameters such as morphology, vasculature, blood flow, and oxygenation in suspect regions can provide more clinically relevant information for diagnostic purposes. “These are all critical steps in moving toward personalized medicine,” he concluded.
Next up was Eva-Marie Sevick-Muraca of Baylor College of Medicine, who discussed her group’s work in NIR fluorescence imaging, specifically for imaging drug responses in vivo. Her group has demonstrated lymph imaging 3 cm deep in animal subjects using NIR fluorescence, which improves upon other molecular-imaging techniques and could allow clinicians to monitor the flow and effect of therapeutic drugs to targeted areas.
Christopher Contag of Stanford University (Stanford, CA) discussed the potential for targeted molecular modeling and imaging to enhance the diagnosis and treatment of cancer, especially as cancer cells evolve in response to existing treatments. “Once you turn off the gene that causes the cancer, the cancer cells begin to regenerate in ways that make them look just like normal cells,” he said. “Sometimes the treatment prompts the relapse. So how do we prevent this?” Among other things, his group is looking at the use of confocal MEMS (microelectromechanical systems) microscopy that can be used in vivo to better image what is taking place at the molecular level in disease development.
Optical-coherence tomography (OCT) was also on the evening’s agenda. Joseph Izatt of Duke University (Durham, NC) described several recent advances in OCT technologies and applications, including volumetric microscopy; new broadband light sources such as Fourier-domain modelocked lasers, and the use of adaptive optics for better volume resolution. He also gave a rundown of some of the more interesting new applications, including 4-D gated OCT imaging of embryonic heart function at Case Western Reserve University (Cleveland, OH) and optical-coherence phase microscopy at Harvard University (Cambridge, MA) and Duke University (Durham, NC).
CARS imaging
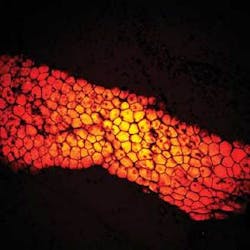
One of the most interesting talks of the evening was given by Xiaoliang Sunney Xie of Harvard University, who discussed his group’s ongoing work with coherent anti-Stokes Raman-scattering (CARS) microscopy (see figure). In fact, CARS-a technique that has been around for at least 30 years and began experiencing a resurgence in the late 1990s-was a “hot topic” throughout this year’s BiOS meeting, with several well-attended presentations on CARS imaging from research groups around the world.
Xie noted that CARS microscopy offers several advantages for noninvasive 3-D imaging of live cells; for example, thanks to improvements in detection sensitivity, better understanding of contrast mechanisms, and the development of new laser sources (such as frequency-doubled modelocked Nd:vanadate combined with an optical parametric oscillator), CARS microscopy can yield high-sensitivity 3-D sectioning. Xie discussed some recent work his group has done with CARS for imaging brain tissue, noting that, while CARS does not have the depth penetration of magnetic-resonance imaging, it does provide better spatial resolution.
About the Author
Kathy Kincade
Contributing Editor
Kathy Kincade is the founding editor of BioOptics World and a veteran reporter on optical technologies for biomedicine. She also served as the editor-in-chief of DrBicuspid.com, a web portal for dental professionals.