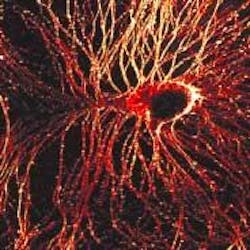
SHG images neuron impulses quickly
Biophysicists from Cornell University (Ithaca, NY) have joined forces with a chemist from Université de Rennes (Rennes, France) to tackle one of the biggest optical-imaging challenges in neuroscience: imaging electrical impulses in the brain. By combining Ti:sapphire laser–based multiphoton microscopy, second-harmonic generation (SHG), and specially developed organic dyes, Daniel Dombeck and Watt Webb of the School of Applied and Engineering Physics at Cornell and Mireille Blanchard-Desce of the Institut de Chimie at Université de Rennes have created high-resolution images of millisecond-scale signaling through nerve cells (see figure).1
While the first demonstration of this technique was in neurons of the lowly sea slug Aplysia, the researchers anticipate that it will eventually be used in brain tissues of higher animals, helping to decipher the wiring of the brain and explain consequences of degenerative brain diseases such as Alzheimer's. In addition to high imaging selectivity and submicron resolution, SHG yields three-dimensional pictures of tissue with minimal damage to living cells.
"One of the big pushes in neuroscience, and the 'holy grail' of techniques, is to optically image neuroactivity," said Dombeck, a graduate student in the Developmental Resource for Biophysical Imaging Opto-Electronics Laboratory. "This technique gives us the ability to look at membrane potential in nerve-cell signaling with high resolution deep in intact tissue, where previous methods were not applicable. We want to image fast electrical signals hundreds of microns into a sample with submicron resolution (up to 400 µm deep), and there has never been a way to do this."
In neuroscience studies, each action potential is a single nerve impulse, traveling through a neuron as chemically gated ion channels open and close with changes in electrical polarity. According to Dombeck, SHG is the first multiphoton technique capable of detecting action potentials and is particularly useful for imaging these impulses because it picks out only the cell membrane where impulses occur and does not suffer from other background signals.
Combined with its fast response to the electrical signals, the selectivity of SHG allows for relatively high signal-to-noise ratio recordings of neuron signaling. Laser light striking molecules of uniform polarity in the focal spot produce a second-harmonic wave of twice the energy, which is easily detected by the microscope in the forward propagating direction. Thus, every change in polarity and every action potential is optically imaged in submicrometer and millisecond spatiotemporal resolution. According to Dombeck, previous attempts in other laboratories to record fast electrical signals in live cells with SHG had achieved at best about 1-s resolution and were not detecting impulses a few milliseconds in duration.
Similar to experiments done at Cornell last summer that involved SHG imaging of the cytoskeleton in nerve cells (see Laser Focus World, September 2003, p. 21), the system used in these latest experiments combines SHG and two-photon-fluorescence (TPF) microscopy in a modified inverted tissue-culture microscope. The excitation source was a modelocked diode-pumped Ti:sapphire laser (100-fs pulses at an 80-MHz repetition rate). The laser polarization was controlled via a Berek polarization compensator and the beam focused into the sample. The resulting SHG emission was collected in a forward-propagating direction, while the TPF was epi-collected through the excitation objective. A combination of dichroic mirrors, bandpass and blue-glass filters, and polarization analyzers separated the signals for detection with photomultiplier tubes. In initial experiments, this system optically recorded action potentials with 0.833-ms temporal and 0.6-µm spatial resolution on soma and neurite membranes.
"We want to know what parts of the brain cells are firing at which time," he said. "There have been linear methods that can do this (fluorescence, absorption, scattering, and birefringence), but they are all limited to somewhat superficial levels of an intact neural system and the resolution was limited. In our approach, the dye generates the SHG signal and when an electrical signal comes by, the properties of the molecule change so that the SHG changes. We did our initial demonstration in a culture dish and we were able to see changes in the SHG that correlated with the electrical signals."
According to Webb, imaging action potentials in living tissue faces two key obstacles: many dyes are chemically toxic to neurons of living animals, and the intense laser pulses can cause phototoxic damage. These obstacles were overcome by using the robust Aplysia neurons, a less-toxic dye, a longer illumination wavelength, and by limiting the duration and intensity of the laser illumination. To use the SHG technique in more-sensitive animal tissues will require a less-toxic dye; according to Dombeck, the most important characteristic of the dye is that it is asymmetric, with all the dye molecules in the membrane pointed in the same direction.
Imaging many neurons
Expanding the imaging from a single neuron to a larger neural network should be relatively easy, Dombeck adds. With the microscope's field of view, he says, it should be possible to record electrical signaling between many neurons at once.
"With submillisecond resolution, we're beginning to see how much the electrical signals can vary between different places of a single neuron," Dombeck said. "With further development, we should be able to see how pathology affects electrical signals. We'd like to know, for example, how much Alzheimer's plaques affect the signal transmission in axons." Other future goals include using this technique to look at very small neural elements such as dendrite spines and moving beyond the current line-scan limitation in the imaging process by using acousto-optic devices rather than conventional galvanometers.
"We need to be able to scan about 1000 times faster than we currently do to really record these signals in order to image hundreds of neurons at once and see what they are doing and how they are interacting," he said. "People have the idea that instead of scanning just one spot, you can scan 100 spots at one time; that would enable you to cover the sample faster."
REFERENCE
- D. Dombeck, M. Blanchard-Desce, W. Webb, J. Neuroscience 24(4) 999 (Jan. 28, 2004).
- K. Kincade, Laser Focus World (September 2003).