Devices based on cathode-ray tubes are the last main vestiges of the vacuum-tube era, and the race to replace them with flat-panel displays is well publicized. However, an emerging technology may leap ahead of its competition with superior cost, performance, and convenience. High-speed full-color displays made from organic light-emitting diodes (OLEDs) can produce brightness and clarity that proponents claim is not available from existing flat-panel displays (see photo). Add to this the possibility of low-cost fabrication on flexible plastic sheets, and a potential market emerges that no other technology can hope to match—imagine folding up your computer monitor and sticking it in your pocket.
Organic is better
Existing flat-panel displays are lit from behind and produce a given color using liquid crystals and filters to subtract the complementary color from the white light. All pixels must be continuously illuminated, even when they appear dark. Organic LEDs do not need backlighting, and are inherently more efficient, thinner, and more robust. Monochrome displays based on this technology are already in use in some consumer electronics. A partnership between Eastman Kodak (Rochester, NY) and Sanyo (Osaka, Japan) demonstrated a prototype 5.5-in. OLED full-color display at Comdex 2000. This year, Sony (Tokyo, Japan) announced a 13-in. diagonal display with SVGA resolution.
The appearance of an OLED display is essentially independent of viewing angle, and equals a standard cathode-ray tube in brightness—both significant advantages over other flat-panel displays. The response time is three orders of magnitude faster than a liquid-crystal display, and OLED displays require less than 10 V to operate. It may even be possible to manufacture large-area displays using an ink-jet printer in place of thin-film vacuum equipment.
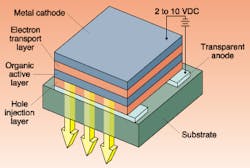
The basic OLED structure consists of a number of thin layers stacked between a transparent anode—most often indium tin oxide (ITO)—and a metallic cathode. The organic layers are typically composed of an electron-transport layer, an active layer, a hole-injection layer, and a hole-transport layer (see Fig. 1). The active layer emits light when forward-biased by a few volts.Organic-light-emitting-diode physics is often discussed in the language of chemistry. The phenomenon of electroluminescence in organic crystals had been observed over 50 years ago, but the crystals were small and difficult to grow, and they required hundreds of volts to produce light. The relatively disordered structure of organic materials limits charge mobility. Charges tend to remain near the electrodes, setting up a space charge that repels further carrier injection. The choice of cathode material is critical in this regard and is an area of ongoing research.
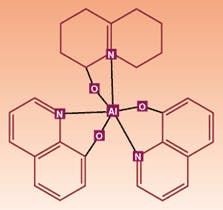
A milestone in organic crystal technology was achieved in the late 1980s by Tang and VanSlyke at Kodak, who developed the first layered device structure. They used the organic crystal hydroxyquinoline aluminum (AlQ; see Fig. 2), which along with its relatives is referred to somewhat quaintly as a "small-molecule" material, in layers less than 100 nm thick and achieving luminescence using only a 10-V forward-bias. Electron- and hole-transport layers were included specifically to move charge carriers away from the electrodes and into the active AlQ layer, where they recombine and emit photons.In the 1970s it was found that some common polymers could be increased in conductivity by a factor of 109. Polymers are normally insulating, but when oxidized (made p-type) or reduced (made n-type with a halogen) and then forward-biased, charged molecules known as polarons and radical cations can migrate along the polymer chain. Alan J. Heeger, Alan G. MacDiarmid, and Hideki Shirakawa were awarded the 2000 Nobel Prize in chemistry for the discovery and development of conductive polymers.
In 1990, a research group at Cambridge University (England) developed a device similar to Tang's and VanSlyke's but made from the polymer polyparaphenylenevinylene (PPV), a key material in modern polymeric OLEDs. Today there is intense and competitive development of devices from both classes of luminous materials—small crystals based largely on AlQ and polymers based largely on PPV.
Tang and VanSlyke, in a separate development, added a dye to increase the luminous efficiency of AlQ. This is essential for producing color displays. The efficiency of an OLED is partly a function of its recombination efficiency and the quantum efficiency of the material. Recombination efficiency is nearly unity—however, quantum statistics for ordinary organic molecules restrict useful light emission to only 25% of the excited states (emission from so-called triplet states to a singlet state is forbidden).
Quantum efficiency in organic crystals is improved dramatically by adding highly fluorescent dyes, similar to those used in dye lasers, to the active layer as triplet state emitters (more elemental dopants are also used). The additive also can determine the wavelength of emission. These same benefits are obtained in polymeric OLEDs by adding different varieties of complex molecules—for instance, the luminous chromophore found in jellyfish has been added to produce orange light. To date OLEDs that span the visible spectrum have been produced.
Can't be too thin or too rich
The emission bandwidth of OLEDs is typically on the order of 50 to 70 nm at full-width half-maximum. Mixing the output of three such red, green, and blue (RGB) diodes will not accurately reproduce an arbitrary color for dynamic full-color displays. The layers are thin enough (<1 µm) so that thin-film interference effects can be used advantageously to narrow the individual emission bandwidth and achieve reliable color reproduction.
An RGB display can be made of OLED pixels by overlapping three colored "sub-pixels" at each pixel location, enabling a higher resolution than having the color sources side-by-side. The upper sub-pixels are transparent to the colors produced underneath them. Trace contaminants from the ITO anode have caused problems with this approach, and there is an effort under way to explore different transparent anodes for stacking structures.
Organic light-emitting diodes are made into both passive-matrix and active-matrix displays. The passive-matrix design is simpler and is already in commercial use for low-cost applications such as cellular phone displays. It is formed by providing each column of pixels in an array with a data signal as the rows of pixels are scanned with a drive current. Rows must be refreshed by the current in a time less than that detectable as flicker by the human eye.
Active-matrix displays are used in high-resolution, high-information applications. They are formed over a pattern of thin-film transistors (TFTs) that is itself deposited on a substrate. The TFT backplane can address each pixel independently, and allows for high speeds and high currents (brightness). This active-matrix structure is ideal for a device in which each pixel is self-illuminating.
A seeming disadvantage to the active-matrix approach is the number of drive transistors required: one for each color at each pixel, plus one to turn the pixel off. For VGA resolution (640 rows x 480 columns), this amounts to millions of TFTs. However, this same technology is already in use in high-resolution liquid-crystal displays. It has even been possible to deposit the TFT backplane over a flexible plastic substrate.
While crystalline organic materials require vacuum deposition onto a glass substrate, it may be possible to layer polymers over this flexible TFT substrate using spin-casting or other inexpensive techniques such as ink-jet printing. The spin-cast process is expected to produce OLED arrays no thicker than a sheet of paper in about two to three hours—much less than the fabrication time of an equivalent inorganic structure.
A little more red, please
Although OLED displays can be as bright as conventional cathode-ray tubes, their overall efficiency of only a few percent has been a problem. Like conventional LEDs, total internal reflection can confine most of the emission inside of the device. It is reasonable to expect that new device structures will greatly reduce these losses.
The luminous efficiencies of OLEDs are measured in candelas per Watt of input. A candela is radiant power factored by the sensitivity of the human eye at the emission wavelength, and as might be expected, OLEDs with emission in the green are most efficient. At the time of this writing, green efficiency is about 1 candela per Watt, red is about a tenth of that, and blue devices are somewhat better. Red efficiency needs to be improved before full-color displays based on OLED technology come to market.
Heat is the enemy of these materials, and maintaining the requisite levels of brightness results in shortened lifetimes. Heat degrades the dyes in the active layer, requiring higher voltage to produce a bright output, and further degrading the organic material. A standard measure of display lifetime is the time for brightness to degrade to 50 candelas per square meter—that is, to about one-half that of a typical monitor. Claims are that prototype displays currently last roughly 10,000 hours.
A device that operates as an LED can in theory be made into a laser. In reality, this can prove difficult: defect densities that are acceptable in LEDs are fatal in laser operation because of the much higher current and photon densities. The relatively disordered crystalline structure of small-molecule materials at first made laser operation seem unlikely. Despite this, there were reports of laser action in organic crystals using laser pump sources.
Organic but coherent
Last year Bell Labs (Murray Hill, NJ) developed an electrically pumped laser made from tetracene, a molecule with four connected benzene rings that form an organic crystal with low self-absorption and high conductivity. Tetracene has proven capable of handling the high current and light densities of laser operation, and is much less expensive than inorganic laser materials such as gallium arsenide.
The Bell Labs laser has a yellow-green emission. So far producing an OLED at new wavelengths has proven far simpler than for an inorganic diode, which requires inventing a new alloy. Researchers at the University of London (England) recently reported OLED emission at 1.5 µm by using erbium in place of aluminum in the AlQ emission layer. Since organic LEDs have already been deposited on and integrated with silicon electronics, the same integration of OLEDs or organic lasers at 1.5 µm may have applications in a future generation of all-optical telecommunications networks.
Next month the series examines advances in excimer lasers and their applications.
About the Author
Stephen J. Matthews
Contributing Editor
Stephen J. Matthews was a Contributing Editor for Laser Focus World.