Lab-on-a-Chip/In Vitro Diagnostics: Facilitating microfluidics with material selection
ALEXIOS P. TZANNIS
Flow cells that shuttle volumes smaller than a drop of blood into functional regions underlie the development, automation, and miniaturization of powerful in vitro diagnostic (IVD) systems. These systems in turn enable a broad range of applications and devices, from digital biology to next-generation genome sequencing, organs-on-a-chip, high-throughput cell-based assays, and high-density multifunctional chips for drug discovery and bioanalysis.
Goals of reducing sample size and reagent consumption, increasing analysis speed, and decreasing cost are pushing IVD device fabrication to new limits while boosting device complexity and functional integration. Their dimensions and complexity present significant challenges, while post-fabrication modifications such as coatings also influence production workflow and cost.
The material used for an IVD device defines not only the patterning and processing applied, but also the manufacturing, performance, functionality, and cost of the device. Thanks to advances in laser machining, photolithography, etching automation, wafer bonding, functionalization, room-temperature UV adhesive bonding, and other processes, glass and glass-hybrid materials are often both the best-performing and most cost-effective consumable materials for IVD devices.
Feature size and design complexity
As dimensions are reduced, surface anomalies once considered defects can become functional, and wafer processing techniques can produce features with dimensions on the same scale as those of biopolymers and cell structures. Reactive-ion etching (RIE) creates cell and biomolecular docking stations in glass, and increased surface roughness can be coupled to a molecular recognition element, such as an aptamer, for increased selectivity. Nanofluidics confines volumes in submicron channels to harness small-scale phenomena.
Though using polydimethylsiloxane (PDMS) with soft lithography is common in academic research, the mechanical, chemical, and optical properties of glass confer many advantages over such materials. For example, wall rigidity and stability help nano- or micro-channels survive bonding or sealing, and the ability of bonded glass to withstand flow pressures is key. And many applications benefit from glass’s thermal properties, as well as its chemical stability and negligible autofluorescence. Finally, glass surface is infinitely tunable with silanization reactions, enabling numerous properties.
While silicon is often the best material for the high-aspect-ratio features needed to confine a single cell or create a specific flow profile, for instance, when other characteristics of glass (e.g., optical smoothness, dielectric constant, metal layer processing step requirements, bonding methods, etc.) are also desired, it is often possible to use glass as a substrate. Processing technology from the semiconductor industry enables creation of high-aspect-ratio micro- and nano-channels, while a process that combines laser micromachining and chemical etching has demonstrated aspect ratios as high as 20:1 in glass. An immense body of work describing the modification of glass surfaces demonstrates that as a substrate, glass has by far the most chemically tunable surface.
Biological applications sometimes call for the integration of optics, optical windows, electrodes, electronics, and three-dimensional structures such as barriers or pores. Using a combination of subtractive techniques such as ultrafast laser fabrication and chemical etching with additive methods such as electroless plating and CVD, it is possible to create complex, multifunctional chips (including those with polymer surfaces) in glass and fused silica—and each material has strengths that benefit specific application and design parameters. Because hybrid-material devices can overcome the challenges involved in using live cells, often the best solution is to leverage the properties of each material.
Choice of material, configuration, and post-fabrication processing for chip-based IVD cell culture systems depends on the cells, media, and phenomena to be observed. Even channel design can affect cell viability, as some cells are more susceptible to shear stress than others. PDMS is often used because its permeability allows for gas exchange among cells on a chip and with the environment surrounding the chip. However, this characteristic can compromise cell health when unintended migration of gases and ions occurs. Similarly, patterning to locate cells in one region of a device has many advantages, but cells may eventually suffer as the surface affects cell shape and physiology.
Neuronal cells are often measured using electrodes, making the standardized patterning of electrodes on glass or silicon a reason to choose either of those materials. The ability to implement transparent indium tin oxide (ITO) electrodes can tip the scales toward glass, as they allow monitoring of cells with a microscope, even on an electrode surface.
Glass is often the best material for optical methods, and it is amenable to room-temperature bonding using UV adhesives, which are well known in industry with verified biotoxicity and biocompatibility data (see Fig. 1).
Cell adhesion and sorting
Methods of confining cells to specific regions include micro- and nano-patterning of surfaces, and use of TMMF, a photostructurable material, to create an intermediary layer of so-called 2.5D structures that capture and culture cells. Another strategy is to use self-assembled monolayers (SAMs) to chemically define surface functionality for adhesion, lubrication, wettability, or protein physisorption. Thiol-based SAMS react with gold, a metal readily patterned on glass. Organosilanes, another powerful tool with well-understood glass-reaction chemistry, enable surface modifications important for IVDs, including increased hydrophobicity for cell/biomolecule adhesion, or droplet/digital microfluidics; increased hydrophilicity to prevent biological material adsorption; and addition of surface charge for ionic association or other purposes.
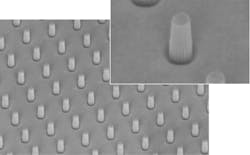
Recent studies have revealed that manipulation of cell geometry, such as confinement to circular or square patterns, affects crucial cellular processes. Deep reactive ion etching (DRIE) can pattern silicon, quartz, or glass wafers for such applications with arrays of silicon micropillars of various geometries and spacing (see Fig. 2).Similarly, biocompatible, photoimageable bonding adhesives (PBA) can enable economical yet complex fluidic channel systems directly on glass using standard MEMS processes.
The need to sort heterogeneous cell populations has broad application, and within the three categories of cell sorting—fluorescence label-based, bead-based, and label-free—are a variety of mechanisms for selection: optical forces, electrokinetic, acoustophoresis, magnetic, mechanical, or passive.
Depending on fluorescence efficiency of the target, wavelength, and limit of detection (LOD), glass or fused silica tends to perform best because of low autofluorescence, even if other materials, such as electrodes, are required. Label-free methods are not necessarily free from the optical transparency and low autofluorescence requirements that drive the choice of glass. If inherent fluorescence, optical, or optoelectronic tweezers are used, then glass or a glass-hybrid is often best.
Genomics applications
As with other bioassays, the best material for high-throughput, small-volume nucleic acid analysis is largely determined by detection mechanism and the materials’ thermal properties. For optical detection, glass is ideal, and as a substrate, it is attractive for other reasons: optical glass or fused silica do not strongly inhibit polymerase chain reaction (PCR) enzymes, nor do they outgas or take on water or ions that can foul enzyme reaction.
A major innovation achieved by the $1000 genome goal was recognition of the role of sample preparation, including amplification and quantitation of DNA libraries using digital PCR (dPCR), a method of separating the sample into single-molecule partitions. Such small partitions make it possible to quantify sequences occurring in small percentages.
The key ability to create thousands to millions of uniformly sized droplets is heavily dependent on design and manufacture of the digital microfluidic chip (see Fig. 3). The resulting precision and selectivity enable understanding of complex expression pathways in diseases such as cancer, and theranostics approaches such as liquid biopsies.Companies such as Advanced Liquid Logic (now part of Illumina), Raindance, and Quantalife (both now part of Bio-Rad) have perfected droplet creation using the geometry and surface chemistry of the microfluidic channels as they interact with droplet-enclosed (hydrophilic) and droplet-enclosing (hydrophobic) fluid.
Glass plays a major role in the manufacture of pressure-driven droplet microfluidic components since it enables such properties as low surface roughness, chemical inertness, and high accuracy of manufacturing tolerances. Additionally, because of the well-known surface chemistry, it is relatively easy to pattern hydrophobic regions on hydrophilic glass to create uniform droplet volumes and assist droplet sorting.
Digital microfluidics uses an array of electrodes and electrowetting to create droplets and control their size and movement. For this, glass is the best material, as the high-density ITO electrodes required are readily patterned on glass (see Fig. 4).Many next-generation sequencing (NGS) technologies use optical methods where the optical properties of glass are leveraged. A key enabling technology in achieving the $1000 genome is the structuring of glass surfaces to create patterned Flow Cells. The patterning enables a significant reduction of the image acquisition time. Important to this success is the accuracy of sub-micron wafer-level patterning, which enables extremely dense packaging of the sequencing space within the microfluidic chip, reaching the optical resolution limit of conventional fluorescence imaging systems.
As the $1000 genome gives way to the $100 genome, the desire to minimize costs by reducing the volumes of expensive reagents drives the limit of detection (LOD) goal further still. This increases the need for low autofluorescence of the substrates of the flow cell, as well as the application of large-scale manufacturing methods.
While label-free assays seem to eliminate the need for glass’s optical properties, glass still offers some advantages. For silicon and glass, submicron control of channel etch depth can improve signal-to-noise ratio for impedance detection. And the ability to readily incorporate ITO electrodes allows integration of digital microfluidic sample preparation, helping to reduce precious sample and expensive reagent consumption.
No matter the application, IVD developers must balance the requirements of biology and device engineering. In every case, the basic step of choosing flow-cell material is hugely impactful.
Alexios P. Tzannis is business development manager, life science at IMT Masken und Teilungen, Greifensee, Switzerland; e-mail: [email protected]; www.imtag.ch/en.