OPTOELECTRONIC APPLICATIONS: INSTRUMENTATION - Next-generation cytometers think outside the box

It has been six years since the first commercial blue solid-state laser (Coherent’s 488 nm diode-pumped Sapphire) entered the market, challenging air-cooled argon-ion lasers in digital imaging and bioinstrumentation. While the Sapphire now boasts more than 5000 installations worldwide, it has, like many of its solid-state competitors, faced some resistance from instrumentation OEMs. While these lasers enable the development of more-compact bioanalysis systems with multispectral capabilities, cost continues to be an issue; although legacy gas-laser systems are much larger and have greater maintenance and replacement costs, solid-state laser sources carry a higher initial price tag and require system manufacturers to reconfigure existing instrument designs and revisit the FDA approval process, which can add one to two years to the product-development cycle.
As a result, high-end spectrometers, flow cytometers, and confocal microscopes are still used primarily by large research laboratories, pharmaceutical companies, and academic facilities. In recent years, however, solid-state lasers have begun to find their way into smaller, less-expensive systems that may not have all the bells and whistles of their predecessors but offer the potential to take flow cytometry and confocal microscopy out of the laboratory and into entirely new markets in the clinical realm-perhaps even to the point of care.
“The solid-state laser is piquing interest among end users partly because these lasers are smaller, more robust, rugged, and a little easier to use than the gas-laser technology,” said William Telford, head of the National Cancer Institute’s NCI ETI Branch Flow Cytometry Core Laboratory, part of the National Institutes of Health (NIH; Bethesda, MD). “But for me the most exciting thing is the variety of wavelengths that are available, from ultraviolet to infrared. There are a lot of fluorescent probes that are not used in flow cytometry because we haven’t had the lasers to excite them. But now there are, and this gives us many more tools to work with in cell biology.”
For example, Telford and colleagues have been experimenting with violet laser diodes (395-415 nm) as an alternative to krypton-ion lasers for cell exploration.1 Violet-excited fluorochromes such as Cascade Blue, Alexa Fluor 405, Pacific Blue, Cascade Yellow, Alexa Fluor 430, and Pacific Orange are important fluorochromes for fluorescent immunophenotyping, while cyan fluorescent protein is one of several fluorescent proteins that require violet excitation. Violet-excited probes are also available for cell cycle analysis, viability assessment, and cell physiology analysis. While krypton-ion lasers can produce several violet laser lines, they are large, expensive, and primarily compatible only with large-scale cell sorters such as the Becton Dickinson FACSVantage, Beckman Coulter Altra, and DakoCytomation MoFlo. According to Telford, the much smaller violet laser diodes are now being integrated into cuvette-based flow cytometers, allowing these more-compact instruments to utilize many of the violet-based probes.
Slowly but surely these lasers are beginning to make their way into the commercial marketplace as well. The FACSAria cell sorter from Becton Dickinson (Franklin Lakes, NJ), for example, features a violet diode laser, blue Sapphire laser, and red HeNe laser. Other products in the company’s FACS product line that now utilize solid-state lasers include the FACSArray, a four-color cytometer that features a red diode laser and green solid-state laser; the FACSCanto, which uses a solid-state blue laser; and the LSR II, a multispectral instrument that can be customized to be all-solid-state, with red diodes replacing the red HeNes and the option of adding a 355 nm frequency-tripled Nd:YAG laser to address the UV spectrum.
“We almost exclusively use solid-state lasers now in our cell-counting systems,” said Bob Hoffman, Fellow at BD Biosciences (San Jose, CA; see Fig. 1). “For the flexibility we need (in creating more compact systems), we are actually more constrained by the electronics, the software, and the number of detectors or photomultiplier tubes needed for each fluorochrome (than the lasers).”
These issues must be addressed by the laser manufacturers and the instrument designers as customers push for increasingly complex multispectral capabilities to be added to flow cytometers. One of the advantages of the legacy gas-laser systems is their ability to address multiple wavelengths; adopting a solid-state laser platform inevitably means multiple lasers must be incorporated into the system to address a customer’s desire for multiple wavelengths.
“At Cobolt we see growing demand from end users for instruments equipped with more excitation lines and at higher power levels, so we foresee a market development in which the more flexible and powerful systems will actually grow in importance,” said Hakan Karlsson, vice president of technology and business development at Cobolt (Stockholm, Sweden). “Therefore, we think that multiline solid-state lasers will be an attractive choice for cytometer manufacturers in the future.”Toward this end, Cobolt has introduced the Calypso line of solid-state lasers. The single-line Calypso offers up to 100 mW at 491 nm, while the Dual Calypso emits simultaneously at 491 and 532 nm in a single beam with 20 + 20 mW of output power (see Fig. 2). Bill Telford at NIH has worked with the Calypso for flow cytometry and confocal microscopy (in April 2006), incorporating the dual-wavelength laser into a BD LSR II, substituting it for the conventional 488 nm source; the results, according to Telford and Karlsson, were very positive.2
“We think Telford’s experiments with our laser were particularly interesting because they showed that the potential problem of the green line contaminating the fluorescence from the blue line could be overcome by simply changing the filter in front of the detector,” Karlsson said. “He used a BD system, put our laser in it, and modified the filter in front of the fluorescein detector so the throughput stopped at 520 nm. This allowed simultaneous excitation in the blue and green with substantially improved resolution for longer-wavelength fluorophores, and that is exactly what we wanted to prove.”
Other end of the spectrum
While several manufacturers have introduced all-solid-state-based diagnostic systems in the last few years, from the laser suppliers’ perspective this is still a drop in the bucket in terms of the overall market potential for these lasers in bioinstrumentation.
“Solid-state lasers are making their way into cytometer systems, although perhaps at a slower rate than expected,” Karlsson said. “More disappointing is the fact that this technology shift does not seem to have sped up the general movement of these systems from lab analysis to clinical diagnostics.”
For example, while Becton Dickinson’s smallest and least-expensive flow cytometer, the FACSCount—which is used for CD4 counting in very resource-poor situations, with about 1000 units now placed in Third World countries, according to Hoffman—still uses a green HeNe, the company has been talking with laser manufacturers for 10 years about developing a reliable, low-cost solid-state replacement for the green HeNe, Hoffman says. In addition, the FACSCalibur still uses an argon laser, and although it would make sense to use a solid-state alternative, Hoffman notes that the argon is still less expensive and redesigning the system to accommodate a solid-state laser would require going back through the FDA approval process-which means the company might ultimately have to raise the price of the system.“Flow cytometry has huge potential to be a real clinical device, but we are still a long way from that,” said Paul Ginouves, director, ion laser systems business at Coherent (Santa Clara, CA). “The laser technology is there, but the equipment manufacturers are in many ways hamstrung because of an end-user requirement for continuity with past technology. It is interesting to consider if we threw out the legacy systems, what would the next generation of flow cytometers look like?”
That is a question being pondered by many in the bioinstrumentation community, from end users to systems developers and even some government officials. On the commercial front, companies such as Luminex, Guava, and Partec have been working hard to develop and market compact, solid-state laser-based cytometers that enable health-care providers to take advantage of what these tools have to offer in terms of clinical diagnostics-not just lab-based analysis (see www.laserfocusworld.com/articles/209783). But these and other product developers are still often handicapped by the cost of the lasers, according to Howard Shapiro, director of the Cytometry Laboratory (West Newton, MA) and a well-known luminary in the field (his book, Practical Flow Cytometry, is considered the reference book for flow cytometry).2
“I’ve been trying to make flow cytometers cheaper for 30 years, and the way to do this is to get rid of the flow,” said Shapiro, who has long championed the notion of “personal cytometers.” “Flow cytometry is, to date, the best technology we have for cell sorting, but around 90% of the flow cytometers now in use don’t sort and don’t need to. A laser-scanning cytometer is slower than a flow cytometer, but you are talking about a very cheap instrument that can do the same things as a flow cytometer and costs significantly less.”
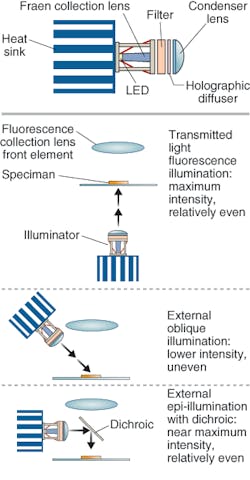
In fact, Shapiro believes that, depending on the complexity of the desired application, it may be possible to use an even less-complex light source: the LED. In various experiments he and his colleagues have shown that it is possible to do single-molecule measurements with an LED-illuminated system and CCD detectors (see Fig. 3).3
“For applications such as AIDS testing in Third World countries where there is little to no infrastructure in terms of the availability of power and clean water, it is advisable that the box be as small, simple, robust, energy-efficient, and inexpensive as possible,” Shapiro said. “Even users in affluent countries would prefer to spend as little as possible on the box. The rapidly improving performance and similarly rapidly decreasing price of high-intensity LEDs and CCD and CMOS imaging chips have made it possible to build apparatus that can do most of the job of a typical benchtop flow cytometer for less than a tenth of the latter’s selling price.”
Shapiro is not alone in his explorations of LED-based cytometry and microscopy. At several scientific meetings in recent years Hoffman has demonstrated the viability of an LED-based flow cytometry system, and he believes the technology has promise-with some tradeoffs, of course.
“It would be impractical for us to take the current FACSCount system and replace it with the LED and go through a complete redesign,” he said. “My experience with LEDs is that, while the intensity of the excitation from a 10 mW LED is about 1% of what I can get with a 1 mW laser, you can illuminate a much larger part of the sample stream because the LED is an extended source. The LEDs create a spot that is 100 to 200 µm high, which means the cells are illuminated for a longer period of time as they go through the excitation spot. So instead of getting only 1% of the total light, you can get 10% because you can illuminate 10 times longer. The tradeoff is that it takes longer to analyze the cell to get a useful amount of collected light for the applications that are of interest. So there are system-level tradeoffs that need to be made but in some applications, such as CD4 counting, this isn’t an issue.”
REFERENCES
1. W. Telford, et al, Cytometry Part A 69A, 1153 (2006).
2. H. Shapiro, Practical Flow Cytometry, Wiley-Liss, 4th edition, ISBN 0471411256.
3. H. Shapiro and N. Perlmutter, Cytometry Part A 69A, 620 (2006).
About the Author
Kathy Kincade
Contributing Editor
Kathy Kincade is the founding editor of BioOptics World and a veteran reporter on optical technologies for biomedicine. She also served as the editor-in-chief of DrBicuspid.com, a web portal for dental professionals.