Diode sources make cancer treatments cost-effective
Diode sources make cancer treatments cost-effective
Growing use of photodynamic therapy and extracorporeal photochemotherapy will boost demand for LED-based medical devices.
Ronald Ignatius and Matthew Ignatius
Traditional light sources for photodynamic therapy (PDT) have been xenon arc lamps or, more frequently, dye lasers pumped by either an argon-ion or frequency-doubled Nd:YAG laser. But diode-based sources--both light-emitting diodes (LEDs) and diode lasers--are beginning to replace traditional sources because the diodes are more convenient and less costly. Researchers at Quantum Devices Inc. (QDI; Barneveld, WI) have pursued the LED route--using funds from two NASA-awarded Phase I Small Business Innovation Research (SBIR) grants--for both PDT and extracorporeal photochemotherapy, another light-based cancer treatment.
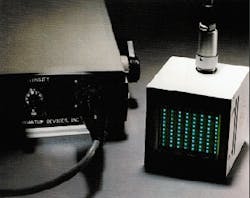
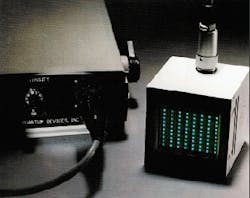
Both of the prototype light sources developed by QDI are undergoing clinical tests. The PDT device has begun human clinical trials on brain tumors in children. The device for extracorporeal photochemotherapy has begun in vitro evaluation in tissue studies (see Fig. 1). Results to date indicate that LEDs are likely to play a major role in providing cost-effective light sources for emerging cancer therapies.
Photodynamic therapy
The need for more-cost-effective light sources for photodynamic therapy of brain tumors is well recognized.1 Each year in the USA about 11,000 new cases of primary brain tumors are diagnosed in children under the age of 16. In children, primary brain tumors are the second most common form of cancer, with solid tumors the most common. The cure rate among children diagnosed with brain tumors is less than 40%. Even though this rate is higher than that for adults, it is still very low. New treatments are being sought as a way to increase survival rates, and one of these new treatments is photodynamic therapy.
Photodynamic therapy (PDT) involves administration of a photosensitive dye that localizes itself in tumor cells and destroys them upon activation with light.2 The first photosensitive agent approved for this application by the US Food and Drug Administration (FDA) is a porphyrin derivative, Photofrin, from QLT PhotoTherapeutics (Vancouver, BC, Canada). The light sources used during the initial phases of PDT research with this drug were lasers that emitted at 630 nm. Those lasers were not practical for routine use, however, because of high cost and lack of reliability. The lack of reliability stemmed from the fact that the lasers used during the initial phases of PDT research had not been designed for such applications, and malfunctions occurred frequently. Another issue with laser light sources is that the narrow bandwidth (2-3 nm) does not take full advantage of the PDT drug absorption characteristics. However, the LED-based probe developed by QDI, for example, has a central wavelength of 688 nm and a wider (30 nm) bandwidth to increase energy absorption by the photosensitive drug.
Emerging alternatives
Although Photofrin is the most frequently used photosensitizer in experimental and clinical studies for brain tumors, several second-generation photosensitizers are under development.3 These photosensitizers exhibit higher peak absorption wavelengths (670- 690 nm) than the first-generation drugs, resulting in deeper penetration of light into the affected tissue.
QDI shipped a working prototype of a neural probe under development for assessing pediatric brain tumors to Dr. Harry Whelan at the Medical College of Milwaukee, WI, for evaluation. During the Phase I activity, Dr. Whelan and his staff were successful in obtaining FDA approval for human clinical trials using Photofrin and the neural probe. One of the important characteristics of the probe is the ability to change the central wavelength of the LEDs to match the absorption wavelengths of many of the second-generation PDT drugs that are now becoming available.
One of the most exciting new drugs is lutetium texaphrin, or Lu-Tex (Pharmacyclics Inc.; Sunnyvale, CA). Preliminary investigations using a 730-nm source with Lu-Tex for treating recurrent breast cancer and malignant melanoma indicate effective photon activation of the drug to a depth of 3 cm in living tissue. Whelan is very interested in investigating Lu-Tex in his research, because the majority of brain stems in children are less than 3 cm in diameter. He is currently in discussions with Pharmacyclics to initiate a dosimetry study in preparation for obtaining FDA approval to use Lu-Tex and the QDI neural probe for human clinical trials.
Throughout the SBIR Phase I PDT effort, NASA has supported the collaboration between Whelan, Pharmacyclics, and QDI. NASA has also supported another Phase I effort at QDI involving extracorporeal photochemotherapy--a treatment that is likely to play an increasingly important role in bone-marrow transplants.
Autologous transplants
During the past two decades, bone-marrow transplantation has evolved from a highly experimental procedure into a fairly routine one. Both the number of medical centers performing transplants and the number of patients receiving them have increased dramatically. Initially, bone-marrow transplants were performed almost exclusively between histocompatible siblings--either genetically identical twins (syngeneic transplants) or genetically different (allogeneic transplants). Much of the recent growth in the field, however, is attributable to the increased use of autologous bone-marrow transplantation for treatment of leukemias and solid tumors.
In an autologous bone-marrow transplant, a small portion of the patient`s own bone marrow is removed during a period of remission and is stored in liquid nitrogen. When cancer recurs after standard therapy, the patient is given escalated (marrow-lethal) doses of radiochemotherapy in an attempt to eradicate the tumor. The cryopreserved marrow is then reinfused to repair the otherwise lethal damage to the bone marrow.
Autologous bone-marrow transplants offer several advantages over allogenic ones. They eliminate the risk of life-threatening graft-versus-host disease; they eliminate the risk of graft rejection; they reduce the incidence of life-threatening viral infections; they obviate the need for histocompatible donors; they are better tolerated by older patients; and they are often the only viable treatment option for patients with small families (no histocompatible sibling donors) or with rare histocompatibility antigen profiles that are underrepresented in donor registries.
Optical purging
One major disadvantage of autologous bone-marrow transplantation is that if a tumor originates in or has metastasized to the bone marrow, there is a significant risk that the autologous bone-marrow transplant graft might be contaminated with live tumor cells. A growing number of bone-marrow transplant centers are, therefore, subjecting autologous marrow grafts to some form of purging before reinfusing them into patients.
Extracorporeal photochemotherapy is a particularly versatile and promising form of marrow purging for leukemia, lymphoma, and neuroblastoma patients. This purging modality adds a photosensitizing dye (Merocyanine 540 from Eastman Kodak (Rochester, NY) to the bone-marrow graft and exposes the cell suspension to an appropriate dose of light. Cells that bind large amounts of the dye, such as leukemia, lymphoma, and neuroblastoma cells, are killed in the process, while cells that bind little or no dye--normal marrow cells--suffer little or no damage.
The procedure has proven highly effective in a preclinical model and has received FDA approval for phase I and II clinical testing. Preclinical and clinical data suggest potentially important roles for extracorporeal photochemotherapy in the prevention and treatment of cancer, graft rejection, graft-versus-host disease, transfusion-related viral and protozoan infections, autoimmune diseases, and rheumatoid arthritis. Some of the applications have obvious implications for NASA`s long-term space missions.
Unlike immunological purging methods, Merocyanine-based photo chemotherapy is effective against a very broad range of leukemias and lymphomas, and the drug costs associated with Merocyanine-based photo chemotherapy are very low--about $10 per graft--in comparison to other pharmacologic or immunologic purging methods--typically more than $1000 per graft. Clinical applications of photochemical marrow-purging methods have, however, been hampered by a lack of suitable light sources and the high costs of consumables (illumination cassettes) that must used in conjunction with certain fluorescent light sources.
Tissue studies
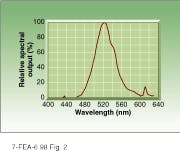
To test LED sources in this application, QDI shipped a 525-nm laboratory prototype to Dr. Fritz Sieber of the Medical College of Wisconsin (Milwaukee, WI) in May 1996. When he received the unit, he immediately started testing for Merocyanine 540 dye absorption (see Fig. 2). His first report indicated that he was achieving photobleaching of the dye at three levels higher than with the fluorescent light source he had been using in his earlier experiments.
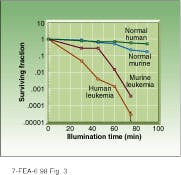
In animal and human tissue studies with the new drug-device combination, Sieber found that normal murine (mouse) cells inoculated with Merocyanine 540 remained virtually unaffected during a 40-minute light exposure, while only 0.01% of similarly inoculated human leukemia cells survived a 75-minute exposure (see Fig. 3). Because photochemotherapy treatments tend to degrade uninfected murine cells more than uninfected human cells, these results indicate that human cells would be less likely to be affected when photosensitized by the device-drug combination under evaluation. These results and those obtained with PDT also indicate that LEDs are likely to play a robust role in the development and deployment of the new light-based cancer therapies. o
ACKNOWLEDGMENTS
The authors thank Dr. Harry Whelan and staff and Dr. Fritz Sieber and staff at the Medical College of Wisconsin for their continuing efforts on these two projects.
REFERENCES
1. M. H. Schmidt et al., J Neurosurgery 84, 552 (1996).
2. H. T. Whelan et al., J Neurosurgery 79, 562 (1993).
3. S. K. Heier and L. M. Heier, "Tissue Sensitizers," Gastrointest. Endosc. Clin. N. Am. 4, 327 (1994).
FIGURE 1. Solid-state light source used to activate photosensitive dye in tissue samples for extracorporeal photochemotherapy is based on a monolithic array of gallium aluminum arsenide (GaAlAs) light-emitting diodes designed to emit diffused monochromatic light.
FIGURE 2. Wide bandwidth of LED light source allows it to maximize energy delivered to photosensitive dye--in this case Merocyanine 540.
FIGURE 3. Extracorporeal photochemotherapy appears to be effective in human and murine (mouse) tissue samples infected with leukemia. In both human and animal samples, uptake of photo sensitive dye in infected cells led to exponential decline in cell population during LED illumination compared to negligible death rate among normal cells.
RONALD IGNATIUS is president and MATTHEW IGNATIUS is a sales manager at Quantum Devices, 112 Orbison St., POB 100, Barneveld, WI 53507.