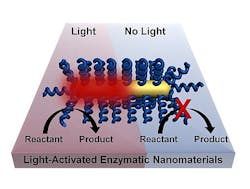
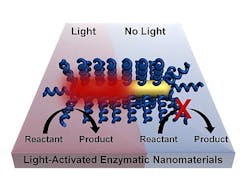
NIR light remotely triggers biochemical reactions
Researchers at Rice University (Houston, TX) are turning light into heat at the point of need, on the nanoscale, to trigger biochemical reactions remotely on demand. Their method makes use of materials derived from unique microbes called thermophiles that thrive at high temperatures, but shut down at room temperature. Possible uses for the new approach include drug manufacture on demand, perhaps from nanoparticle-infused tattoos on the body, or for lowering blood sugar concentrations as a different way to manage diabetes.
The project, led by postdoctoral fellow Matthew Blankschien and graduate student Lori Pretzer, combines enzymes from thermophiles with plasmonic gold nanoparticles that heat up when exposed to near-infrared (NIR) light. That activates the enzymes, which are then able to carry out their functions.
This effectively allows chemical processes to happen at lower temperatures. Because heating occurs only where needed--at the surface of the nanoparticle, where it activates the enzyme--the environment stays cooler.
âBasically, weâre getting the benefits of high-temperature manufacturing without needing a high-temperature environment,â explains Blankschien, who won the Peter and Ruth Nicholas Postdoctoral Fellowship two years ago to work on these ideas. âThe challenge was to keep the higher temperature at the nanoparticle, where the enzyme is activated, from affecting the environment around it.â
The particle at the center of the process is a gold nanorod about 10 nm wide and 30 nm long that heats up when hit with NIR laser light. The rods are just the right size and shape to react to light at around 800 nm. The light excites surface plasmons that ripple like water in a pool, in this case emitting energy as heat.
The new research, however, heats nanoparticles draped with a model thermophilic enzyme, glucokinase, from Aeropyrum pernix. A. pernix is a microbe discovered in 1996 thriving near hot underwater vents off the coast of Japan. At around 176 degrees Fahrenheit, A. pernix degrades glucose, a process necessary to nearly every living thing. The enzyme can be heated and cooled repeatedly.
In their experiments, Blankschien and Pretzer cloned, purified, and altered glucokinase enzymes so they would attach to the gold nanoparticles. The enzyme/nanoparticle complexes were then suspended in a solution and tested for glucose degradation. When the solution was heated in bulk, they found the complexes became highly active at 176 degrees, as expected.
Then, the complexes were encapsulated in a gel-like bead of calcium alginate, which helps keeps the heat in but is porous enough to allow enzymes to react with materials around it. Under bulk heating, the enzymesâ performance dropped dramatically because the beads insulated the enzymes too well.
But when encapsulated complexes were illuminated by continuous, NIR laser light, they worked substantially better than under bulk heating while leaving the solution at near-room temperature. The researchers found the complexes robust enough for weeks of reuse.
The work appears in the American Chemical Society journal ACS Nano; for more information, please visit http://pubs.acs.org/doi/abs/10.1021/nn3048445.
-----
Follow us on Twitter, 'like' us on Facebook, and join our group on LinkedIn
Laser Focus World has gone mobile: Get all of the mobile-friendly options here.
Subscribe now to BioOptics World magazine; it's free!