CANCER MICROSURGERY: Taking on tiny tumors
Used alone, lasers and other optical techniques have significant value in diagnosing and treating cancers at the microscopic level. When combined with other technologies, that value increases synergistically. Three recent examples show the medical benefits of doubling up on microtechnologies. Experimental studies that use combinations of nanoparticles and light, laser microsurgery and two-photon microscopy, and scanning technology and microchips have shown promise in identifying or even killing tiny tumor cells.
Stimulating effect of quantum dots
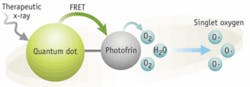
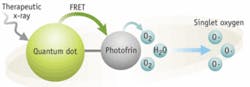
A team at the University of Virginia has developed a method of killing tumor cells that relies on quantum dots. These semiconductor nanostructures typically emit light when exposed to ultraviolet radiation. Wensha Yang, an instructor in the University of Virginia’s radiation oncology department, realized that megavoltage x-rays, such as those used in cancer radiotherapy, have the same stimulating effect on quantum dots. That makes the dots good candidates for use in therapies that apply light-activated compounds to treat cancer.
The key to the approach is Photofrin, a photosensitizer readily absorbed by cancer cells that becomes chemically active on exposure to light; that activity kills the cells that have absorbed the compound. Yang and his colleagues reasoned that combining quantum dots with the Photofrin could make the process more efficient. That would allow it to kill even deeply seated cancer cells. Once the Photofrin–quantum-dots combination reaches a tumor, a three-stage process starts. A burst of powerful radiation causes the quantum dots to emit light. That light stimulates the Photofrin’s chemical activity. And the activity kills the cancer cell.
The technique minimizes damage to healthy tissue in two ways: by activating the Photofrin only when it has reached a tumor, and by targeting the tumor with conformal radiation that contains the impact of the Photofrin. “As a result,” Yang says, “the toxicity of the drug is substantially lower in the lower radiation-dose area” beyond the tumor. So far, Yang reported at the annual meeting of the American Association of Physicists in Medicine, the team applied the method only to cancer cells grown in culture. But in theory, he added, the technique could work on tumors too deep in the body to be reached by an external source of light.
Preferential destruction of cancer cells
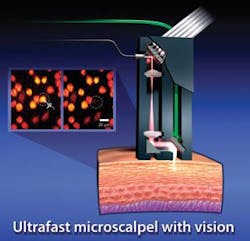
Another group uses a different method to preferentially destroy cancer cells. The group uses an image-guided femtosceond laser to ablate single cells and subcellular structures with high precision. This technology also permits clinicians to remove individual tumor cells without damaging contiguous healthy cells. It does so because the laser’s pulses sear a targeted cell so quickly and accurately that the heat can’t escape to affect neighboring healthy cells. “You can remove a cell with high precision in 3-D without damaging the cells above and below it,” says Adela Ben-Yakar of the University of Texas, who worked on the method with electrical engineer Olav Solgaard of Stanford University.
Because commercially available femtosecond systems are too bulky, Ben-Yakar and Solgaard developed their own version that incorporates a miniaturized scanning mirror, a fiber-optic cable that can withstand powerful light pulses, and a flexible probe that can focus the light on spots smaller than human cells. To reduce damage to the fiber, the team stretched the laser pulses out before they traveled through the fiber, and reconstructed them into more intense, shorter pulses before they entered the tissue.
To test the system, the team reports in Optics Express, Ben-Yakar directed laser pulses at breast-cancer cells inside biostructures that mimic the optical properties of breast tissue. She has also proved the technology with laboratory-grown structures that mimic skin and other tissues. If the method fulfills its early promise, it could find application in destroying small tumors of the vocal cords, cancer cells left in the body after the surgical removal of solid tumors, and individual cancer cells inside the human body.
Ben-Yakar is also exploring the use of gold nanoparticles to magnify the effect of laser light on tumor cells. “If we can consistently deliver nanoparticles to cancer cells or other tissues that we want to target, we would be able to remove hundreds of unwanted cells at once using a single femtosecond laser pulse,” she says. “But we would still be keeping the healthy cells alive.”
Scanning for genetic signatures
A group at the Massachusetts General Hospital (MGH), meanwhile, has created a microchip scanner that detects and analyzes tumor cells in the bloodstream. The so-called CTC-chip can detect cancer cells in the blood at a concentration of one in a billion. The device can also determine the genetic signatures of tumors, thereby permitting physicians to identify those appropriate for specific treatments.
“The CTC-chip opens up a whole new field of studying tumors in real time,” explains Daniel Haber, director of the MGH Cancer Center and senior author of a report in the New England Journal of Medicine. “When the device is ready for larger clinical trials, it should give us new options for measuring treatment response, defining prognostic and predictive measures, and studying the biology of blood-bone metastasis, which is the primary method by which cancer spreads and become lethal.” –PG