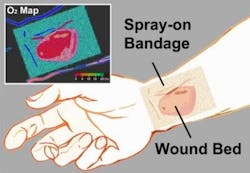
Phosphorescent glow from paint-on 'smart' bandage measures tissue oxygenation
Inspired by a desire to help wounded soldiers, an international, multidisciplinary team of researchers led by assistant professor Conor L. Evans at the Wellman Center for Photomedicine of Massachusetts General Hospital (MGH) and Harvard Medical School (HMS), both in Boston, MA, has created a paint-on, see-through "smart" bandage that glows to indicate a wound's tissue oxygenation concentration. Because oxygen plays a critical role in healing, mapping these levels in severe wounds and burns can help to significantly improve the success of surgeries to restore limbs and physical functions.1
The new bandage provides direct, noninvasive measurement of tissue oxygenation by combining three simple, compact and inexpensive components: a bright porphyrin-dendrimer phosphor sensor molecule with a long phosphorescence lifetime and appropriate dynamic range; a bandage material compatible with the sensor molecule that conforms to the skin's surface to form an airtight seal; and a camera-based imaging device capable of capturing the oxygen-dependent signals from the bandage with high signal-to-noise ratio.
Green-to-red color map
To make the bandage simple to interpret, a green oxygen-insensitive reference dye was also incorporated into the bandage so that changes in tissue oxygenation are displayed as a green-to-red color map.
The bandage is applied by painting it onto the skin's surface as a viscous liquid, which dries to a solid thin film within a minute. Once the first layer has dried, a transparent barrier layer is applied atop it to protect the film and slow the rate of oxygen exchange between the bandage and room air, making the bandage sensitive primarily to the oxygen within tissue.
The final piece involves a camera-based readout device, which performs two functions: it provides a burst of excitation light that triggers the emission of the phosphors inside the bandage, and then it records the phosphors' emission.
"Depending on the camera's configuration, we can measure either the brightness or color of the emitted light across the bandage or the change in brightness over time," says lead author Zongxi Li, an HMS research fellow on Evans' team. "Both of these signals can be used to create an oxygenation map." The emitted light from the bandage is bright enough that it can be acquired using a regular camera or smartphone, opening up the possibility of a portable, field-ready device.
Immediate applications for the oxygen-sensing bandage include monitoring patients with a risk of developing ischemic (restricted blood supply) conditions, postoperative monitoring of skin grafts or flaps, and burn-depth determination as a guide for surgical debridement (the removal of dead or damaged tissue from the body).
Next steps for the technology
"We're developing brighter sensor molecules to improve the bandage's oxygen sensing efficiency," says Emmanuel Roussakis, another research fellow in Evans' laboratory and co-author, who is leading the sensor development effort. The team's laboratory research will also focus on expanding the sensing capability of the bandage to other treatment-related parameters such as pH, bacterial load, oxidative states, and specific disease markers, and on incorporating an on-demand drug-release capacity.
The team is actively searching for industry partners. Research support came from from the Military Medical Photonics Program from the U.S. Department of Defense and from the National Institutes of Health.
REFERENCE:
1. Z. Li et al., Biomedical Optics Express, Vol. 5, Issue 11, pp. 3748-3764 (2014); http://www.opticsinfobase.org/boe/abstract.cfm?uri=boe-5-11-3748
About the Author
John Wallace
Senior Technical Editor (1998-2022)
John Wallace was with Laser Focus World for nearly 25 years, retiring in late June 2022. He obtained a bachelor's degree in mechanical engineering and physics at Rutgers University and a master's in optical engineering at the University of Rochester. Before becoming an editor, John worked as an engineer at RCA, Exxon, Eastman Kodak, and GCA Corporation.