Artificial retina from Second Sight gets FDA approval
Arlington, VA--The first bionic eye using artificial retina technology has been granted market approval for patients in the United States by the U.S. Food and Drug Administration (FDA). The prosthetic technology was developed in part with support from the National Science Foundation (NSF) by Second Sight Medical Products (Sylmar, CA)--part of the team of scientists and engineers from the university, federal, and private sectors who spent nearly two decades developing the artificial retina system with public and private investment.
The device, called the Argus II Retinal Prosthesis System, transmits images from a small, eye-glass-mounted camera wirelessly to a microelectrode array implanted on a patient's damaged retina. The array sends electrical signals via the optic nerve, and the brain interprets a visual image.
The FDA approval currently applies to individuals who have lost sight as a result of severe to profound retinitis pigmentosa (RP), an ailment that affects one in every 4,000 Americans. The implant allows some individuals with RP, who are completely blind, to locate objects, detect movement, improve orientation and mobility skills and discern shapes such as large letters.
The researchers' efforts have bridged cellular biology--necessary for understanding how to stimulate the retinal ganglion cells without permanent damage--with microelectronics, which led to the miniaturized, low-power integrated chip for performing signal conversion, conditioning and stimulation functions. The hardware was paired with software processing and tuning algorithms that convert visual imagery to stimulation signals, and the entire system had to be incorporated within hermetically sealed packaging that allowed the electronics to operate in the vitreous fluid of the eye indefinitely. Finally, the research team had to develop new surgical techniques in order to integrate the device with the body, ensuring accurate placement of the stimulation electrodes on the retina.
The external camera system--built into a pair of glasses--streams video to a belt-worn computer, which converts the video into stimulus commands for the implant. The belt-worn computer encodes the commands into a wireless signal that is transmitted to the implant, which has the necessary electronics to receive and decode both wireless power and data. Based on those data, the implant stimulates the retina with small electrical pulses. The electronics are hermetically packaged and the electrical stimulus is delivered to the retina via a microelectrode array.
"The retinal implant exemplifies how NSF grants for high-risk, fundamental research can directly result in ground-breaking technologies decades later," said Acting NSF Assistant Director for Engineering Kesh Narayanan. "In collaboration with the Second Sight team and the courageous patients who volunteered to have experimental surgery to implant the first-generation devices, the researchers of NSF's Biomimetic MicroElectronic Systems Engineering Research Center are developing technologies that may ultimately have as profound an impact on blindness as the cochlear implant has had for hearing loss."
"The artificial retina is a great engineering challenge under the interdisciplinary constraint of biology, enabling technology, regulatory compliance, as well as sophisticated design science," adds Wentai Liu. "The artificial retina provides an interface between biotic and abiotic systems. Its unique design characteristics rely on system-level optimization, rather than the more common practice of component optimization, to achieve miniaturization and integration. Using the most advanced semiconductor technology, the engine for the artificial retina is a 'system on a chip' of mixed voltages and mixed analog-digital design, which provides self-contained power and data management and other functionality. This design for the artificial retina facilitates both surgical procedures and regulatory compliance."
SOURCE: The National Science Foundation; http://www.nsf.gov/news/news_summ.jsp?cntn_id=126756&org=NSF&from=news
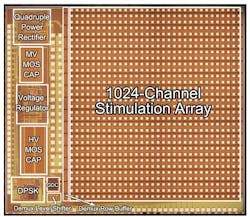
IMAGE: While the Argus II is a major breakthrough in retinal prosthetics, researchers are continuing their research. This third-generation retina chip, itself still very early in the development stage, contains 1000 electrodes and was developed by Wentai Liu, a professor of bioengineering at the UCLA Henry Samueli School of Engineering and Applied Science and his colleagues. Early engineering done by Liu and his team was licensed to Second Sight for the Argus II Retinal Prosthesis System. (Courtesy Wentai Liu, UCLA)