Optical Coherence Tomography/Ophthalmology: OCT angiography: A new approach with 'gold standard' capabilities and more
CHIEH-LI CHEN, QINGIN ZHANG, ANQI ZHANG, and RUIKANG K. WANG
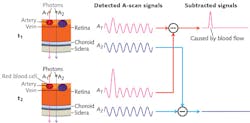
Since its first demonstration in a biomedical application in 1991, optical coherence tomography (OCT)1 has become an indispensable tool in routine clinical practice for anatomical (structural) imaging and therapeutic monitoring—especially in ophthalmology.2 Recent advances in light sources and detection techniques have enabled OCT to advance and extend dramatically, to the point of enabling functional imaging. Now, a newly developed technique, OCT angiography (OCTA), is allowing the functional visualization of blood vessel networks in the eye. Inheriting all the advantages of OCT—such as the ability to generate cross-sectional images of biological structures in three dimensions (3D) with high sectioning resolution (1-10 μm)1—OCTA can provide functional information about the dynamics of blood vessel networks in vivo, without exogenous intravenous dye-injection.3
The concept of OCTA is to use changes in OCT signals caused by moving particles (for example, red blood cells) as the contrast mechanism for imaging functional flows. To give a brief description of its operation, imagine two OCT signals—one backscattered from static structural tissue and the other backscattered from moving particles (such as red blood cells) in vessels. The signal from structural tissue remains steady, while the signal from flowing blood changes over time. To differentiate the moving particles from static tissue, repeated cross-sectional scans (B-scans) are performed at the same location. Temporal changes of the OCT signal in adjacent scans caused by moving particles generate the angiographic contrast and allow visualization of the microvasculature in OCTA (see Fig. 1).
Compared to the 'gold standards'
Compared with other functional imaging techniques, a key advantage of OCTA is that it is non-contact and noninvasive. Fluorescein angiography (FA) and indocyanine green angiography (ICGA) are the current "gold standards" for diagnosing vasculature abnormality in clinical practice. However, the invasiveness of the dye injection combined with possible adverse reactions, such as nausea or anaphylactic response in some rare cases, makes them unsuitable for widespread ophthalmic screening applications and for frequent monitoring. By contrast, OCTA enables visualization of ocular vessels by detecting the differences between cross-sectional scans without intravenous contrast agent or dye injection. Thus, OCTA may be a better alternative in longitudinal disease monitoring-especially for progressive diseases showing high correlation with vessel dysfunction (such as age-related macular degeneration, diabetic retinopathy, and glaucoma) that may benefit from early intervention or treatment.
In addition, OCTA is more time-efficient. It takes 10–30 min. for FA and ICGA imaging, while only a few seconds for OCTA to complete one volumetric scan. Thirdly, OCTA provides visualization of in vivo vasculature in 3D with microscopic resolution. Though FA and ICGA provide images with a wide field of view, the images are two-dimensional and therefore cannot provide depth information of vasculatures. As a depth-resolved imaging technique, OCTA allows visualization of vessel networks at various depths. These vessel networks can be segmented into specific retinal and choroidal layers to determine where the disease originates. Furthermore, the high axial and transversal resolution of OCT systems enable detection of capillaries and visualization of microcirculation.
Data processing
Increased interest in OCTA has spawned the development of numerous data-processing algorithms. To classify them, the basic concept of OCT signal should be introduced: Raw data in Fourier-domain OCT consists of spectral interferograms captured by the detector. Fourier transformation is applied to the raw data to obtain depth-resolved signals. After Fourier transformation, the OCT signal contains both magnitude and phase information, which have both been explored, individually and together, to develop angiographic methods for the purpose of contrasting blood flow within living tissue. Therefore, the various OCTA approaches can be roughly categorized, but not limited into three groups:
1. OCTA based on both the magnitude and phase of OCT signal (complex signal);
2. OCTA based on the magnitude of OCT signal; and
3. OCTA based on the phase of OCT signal.
Optical microangiography (OMAG)-based OCTA is one of the leading techniques, employing the change of complex signal (both intensity and phase changes) to contrast blood flow information. OMAG has been shown to have ultra-high sensitivity to the microcirculation.3,4 OMAG algorithm has been implemented both in a 100 kHz 1 μm swept-source OCT (SS-OCT) prototype and a CIRRUS HD-OCT 5000 system (both by Carl Zeiss Meditec, Dublin, CA) to conduct clinical evaluations. The CIRRUS HD-OCT 5000 system with AngioPlex OCT angiography, which uses the OMAG algorithm, operates at a central wavelength of 840 nm and a speed of 68,000 A-scans/s, and is the first and only OCTA technology to have received clearance from the U.S. Food and Drug Administration (FDA).
A key feature of CIRRUS HD-OCT 5000 is an active eye-tracking mechanism able to trace and compensate eye motion in real time, and provide motion-artifact-free angiographic images.5 It allows wide-field imaging (>10 × 10 mm2) without sacrificing resolution (that is, the same spatial sampling rate as in the typical 3 × 3 mm2 field of view), which increases effectiveness of OMAG for clinical practice.
Application in human studies
OMAG has been widely applied in human eye diseases to provide more information about retinal and choroidal circulation. Several studies were conducted using both a CIRRUS HD-OCT 5000 prototype with active motion-tracking capability and a prototype 1 μm SS-OCT system (with center wavelength at 1060 nm).6 For the CIRRUS HD-OCT 5000 prototype, a montage scan protocol enabled by motion-tracking capability centered at the fovea was used to acquire volumetric datasets with 10% area overlap between adjacent dataset for later montaging.5 For the SS-OCT prototype, a single volumetric scan was acquired from the subjects. Four consecutive B-scans along the transverse direction were acquired at each fixed longitudinal location before the scanning probe proceeded to the next location along the longitudinal direction. A sampling interval of approximately 9.8 μm was achieved in both directions to guarantee high-resolution angiograms. The time difference between two adjacent B-scans was approximately 3.6 ms. A single-volume dataset can be acquired in ~4 s.
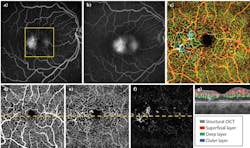
For later data processing, OMAG uses a complex value differentiation algorithm to extract in vivo blood flow information. Retinal layer segmentation can be performed to isolate specific retinal and choroidal layers. Typically, three main retinal layers are segmented: a superficial retinal layer (SRL) that includes the ganglion cell layer and the inner plexiform layer; a deep retinal layer (DRL) that includes the inner nuclear layer and the outer plexiform layer; and an outer retinal layer (ORL) that includes the outer nuclear layer and the external limiting membrane. The segmentation can also be used to separate the choroid into choriocapillaris and deep choroid layers. The segmented layers can be adjusted to provide a better presentation of the vasculature for various ocular pathologies. Finally, the en face vascular images are created by using a maximum (or average) projection method within the layer of interest and then used to correlate the findings between OMAG and conventional methods (FA/ICGA images).
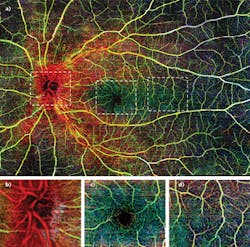
Figure 2 shows a wide-field OMAG angiogram enabled by real-time motion-tracking of normal retinal vasculature, captured with CIRRUS HD-OCT 5000 system.5 Three layers were segmented to give a better demonstration according to depth. Figure 2a shows the results of the wide-field OMAG angiogram (~12 × 16 mm2), including nerve fiber layer (NFL), SRL, and DRL.The vascular en face images from OMAG are comparable to the current gold standard, and further provide angiograms with high resolution and the depth information of pathologies. Though currently there is no standard way of evaluating OCT angiography and new clinical endpoints must be established with larger studies, OMAG has demonstrated its potential as being a useful and powerful tool for diagnosis, detection, and monitoring of various ocular pathologies.
REFERENCES
1. D. Huang et al., Science, 254, 1178–1181 (1991).
2. C. R. Baumal, Curr. Opin. Ophthalmol., 10, 182–188 (1999).
3. R. K. Wang, IEEE J. Sel. Top. Quantum Electron., 16, 545–554 (2010).
4. R. K. Wang, L. An, P. Francis, and D. Wilson, Opt. Lett., 35, 9, 1467–1469 (2010).
5. Q. Zhang et al., J. Biomed. Opt., 20, 066008 (2015).
6. M. R. Thorell et al., Ophthalmic Surg. Lasers Imaging Retina, 45, 369–380 (2014).
Chieh-Li Chen, Ph.D., Qinqin Zhang, Ph.D., and Anqi Zhang, Ph.D., are senior research fellows, and Ruikang K. Wang, Ph.D., is a professor, all in the Bioengineering & Ophthalmology Department at the University of Washington, Seattle, WA; e-mail: [email protected]; http://depts.washington.edu/wangast.