Near-infrared photodetectors enable new applications
Field-assisted photoemission at longer wavelengths has been studied for decades. Yet while the materials under study emitted electrons into a vacuum when irradiated by IR light, they were not used in commercial products. In the late 1990s, however, researchers at Hamamatsu Photonics Central Research Lab developed field-assisted photocathodes based on indium gallium arsenide phosphide (InGaAsP) that could be reliably made into detectors and achieve reasonable quantum efficiencies. Quantum efficiency (QE), the intrinsic ability of a material to convert light into electrons, is measured in terms of the percentage of incident photons converted to electrons and detected as output.
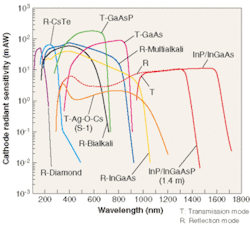
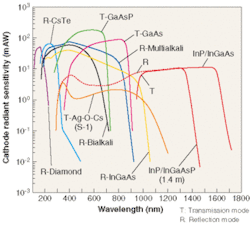
Using the newly developed technology, a photomultiplier tube (PMT) was created based on two different field-emission photocathodes. The first photocathode uses InP/InGaAsP and has a spectral response range of 300 to 1400 nm, whereas the second uses InP/InGaAs and has a spectral response range of 300 to 1700 nm (see Fig. 1).
Photomultipliers are vacuum tubes consisting of a photocathode (photoemissive surface), dynodes (secondary electron-emitting surface), and an anode (electron-collection electrode). When light enters the window of the PMT, it strikes the photocathode, which, via the photoelectric effect, emits an electron into the vacuum. The electron is accelerated by an electric field created by applying voltage to the various electrodes. The accelerated electron is focused onto the first dynode where it creates secondary electrons through the energy of its impact. These electrons are then focused onto the second dynode, where the process repeats itself. A typical PMT consists of 9 to 12 dynode stages and has an electron gain of 500,000 to 10 million.
What makes PMTs based on InGaAsP field-assisted photocathode technology unique is the combination of a solid-state photocathode with near-IR sensitivity coupled to the virtually noise-free amplification of a photomultiplier tube. Two of the most popular solid-state detectors currently being used are InGaAs and germanium photodiodes. These devices achieve QE greater than 60% at the peak wavelength. To be useful as detectors, however, photodiodes must be coupled to amplifiers. And in very low-light applications the signal-to-noise ratio of many solid-state detector/amplifier combinations can be rendered insufficient by high amplifier noise. Even when devices are cooled to liquid-nitrogen temperatures and the light signal is integrated over a period of seconds, the signal-to-noise ratio of commonly used photodetector technology can remain marginal for numerous applications.
While near-IR PMTs have somewhat lower QE than many detector/amplifier combinations, the almost noise-free gain of the dynodes (noise figure 1.2 to 1.4) makes them suitable for counting single photons in the near-IR wavelength ranges. Furthermore, the very high gain bandwidth (1 × 1014 Hz) of PMTs makes them suitable for high-speed timing measurements in the near-IR at very weak light intensities. Therefore, the near-IR PMT is well suited for applications with very low light levels or low light levels that require timing information.
Photodynamic therapy
One such application is photodynamic therapy (PDT), a minimally invasive treatment for cancer or wet macular degeneration that works by injecting a "photosensitizer" (believed to concentrate in the malignancy) or by applying the photosensitizer topically to the area of interest. The photosensitizer is then illuminated with the appropriate wavelength of light for that particular compound. Once illuminated, the photosensitizer is activated from its ground state to its excited singlet state. It then transfers to the triplet state by intersystem crossing, and, finally, transfers its energy to oxygen in the triplet state (3O2). The result is generation of active singlet oxygen (1O2).
Singlet oxygen is the suspected cytotoxic agent in PDT. The strong oxidizing properties of singlet oxygen damage the DNA, mitochondria, or lipid membranes, causing the malignant cell to die. Because the singlet oxygen is only generated when exposed to light, it is possible to localize the effect of the photosensitizer and not damage healthy cells. Patients must remain out of direct sunlight for some period of time to avoid complications from the PDT treatment.
In this application, the near-IR PMT monitors the luminescence at 1270 nm when the singlet oxygen decays to the triplet ground state.1 Before the advent of the near-IR PMT, it was nearly impossible to monitor the effects of PDT in a biological sample because of the very weak emission and very short lifetime of the singlet oxygen (tens of microseconds; see Fig. 2).
The near-IR PMT might also be used for PDT dosimetry, in which the amount of 1270-nm luminescence helps determine the proper amount of photosensitizer to use in a particular case.2 The amount of 1270-nm luminescence can be used to determine whether the tumor is hypoxic, which is important in determining the effectiveness of PDT. The near-IR PMT is also being used to conduct research into new photosensitizing compounds.
Raman spectroscopy
Similar photodetection principles are proving useful for Raman spectroscopy—which has shown promise for in vivo detection of cancer—because vibrational Raman spectra are highly specific in detecting molecular structure.3 This makes Raman spectroscopy potentially suitable for distinguishing between healthy tissue and malignant tissue. But Raman scattering is a second-order scattering effect, and therefore, the Raman signals are very weak.
In traditional Raman systems, a sample is illuminated by a laser (emission wavelength greater than 800 nm). The Raman-shifted spectra is emitted at a wavelength longer than the irradiation wavelength and detected by a sensitive charged-coupled-device (CCD) imaging array that can integrate the Raman spectra across many wavelengths simultaneously. Difficulties arise when trying to do Raman spectroscopy on human tissue samples, however, because the tissue has a tendency to autofluoresce—that is, to emit light from compounds in the tissue sample. The autofluorescence can be many times stronger than the Raman emission, thereby drowning out the Raman signal. The further into the infrared the excitation laser is shifted, the less the contributions from autofluorescence. The spectral response of CCDs does not extend past 1100 nm, however, so they cannot entirely avoid the autofluorescence problem.
Very good results have been obtained gathering Raman spectra using an image intensifier with an InP/InGaAsP photocathode. The Raman spectra not only allow cancerous cells to be distinguished from healthy cells, but may even determine the type of cancer.
An image intensifier consists of a photocathode, microchannel plates, and a phosphor screen. Light enters the input window and is converted to an electron by the photocathode. The electrons are then accelerated into a microchannel plate consisting of millions of 12-µm pores with walls made of a secondary electron-emitting material.
When an electron enters a pore, it strikes the sidewall, which produces electrons that are accelerated down the pore, where they again strike the sidewall to produce more electrons. The process continues along the full length of the pore. The resulting amplification is about 1000× per plate and image intensifiers typically contain one to three plates. Once the electrons leave the microchannel plate, they are accelerated into a phosphor screen that produces light. Image intensifiers convert light at one wavelength to electrons, then amplify it and convert it to visible light. The same technology is used in night-vision equipment.
The advantage of the near-IR image intensifier over a near-IR PMT is that it can image the entire Raman spectra at one time. The image intensifier can acquire the Raman spectra in about one minute, whereas the PMT would take hours because each wavelength must be individually scanned into the PMT.
Materials research
The near-IR PMT has shown promise in materials research as well. It has been used successfully in monitoring the photo-luminescence from silicon wafers to characterize the nonuniformities of ultrathin silicon-on-insulator (SOI) wafers.4, 5 These SOI wafers may be the next-generation substrate for ultra-large-scale integrated circuits. The uniformity is measured by rastering an excitation laser across the wafer surface, collecting the resulting photoluminescence with an objective lens, and passing it through a 1050- to 1230-nm bandpass filter. The images generated reveal nonuniformities in layer thickness, microdefect distributions, and so on. The near-IR detectors have also been used to study the photoluminescent properties of quantum dots.
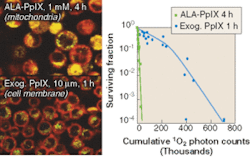
FIGURE 3. Survival of human tumor cells has been observed as a function of singlet oxygen (1O2) generated during photodynamic therapy. Confocal fluorescence microscopy shows the different localization of the photosensitizer protoporphyrin IX (PpIX) synthesized in the cells by incubating with aminolevulinic acid (ALA) or by incubating the cells with PpIX itself (left). To assess cell survival versus total 1O 2 generated, 1O 2 was measured in cell suspensions directly by its near-IR luminescence emission at 1270 nm using a Hamamatsu R5509-42 photomultiplier tube operating in time-resolved, single-photon counting mode. The curves show the high phototoxicity when the photosensitizer is localized in the mitochondria rather than the cell membrane (right).
Overall, near-IR detectors have opened new areas of research that were previously nearly impossible. The reasonable QE coupled with the virtually noise-free gain make single-photon photodetection and imaging possible. The energy bandgap of the cathode is very small, so cooling of the detectors is necessary. Liquid nitrogen or multistage Peltier is required to achieve the appropriate cooling temperature of -60°C (see Fig. 3). The applications for which this detector is suitable represent a niche in the photodetector market characterized by a need for state-of-the-art, time-resolved light detection with resolution less than 100 ps or the counting of photons in the near-IR.
EARL HERGERT is an applications engineer at Hamamatsu, P.O. Box 6910, Bridgewater, NJ 08807-0910; e-mail: [email protected].
REFERENCES
- M. Niedre, M. S. Pattersonl, B. C. Wilson: Photochemistry and Photobiology 75 (2002).
- T. Hirano et al, J. Photoscience 9 (2002).
- S Kaminaka et al., J. Raman Spectros. 33 (2002).
- M. Tajima etal., Jpn. J. Appl. Phys. 43 (2004).
- M. Tajim, S. Ibuka: J. of Appl. Phys. 84(4) (1998).